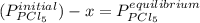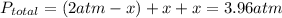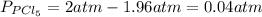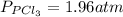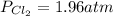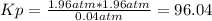Chemistry, 10.10.2019 18:10 lailabirdiemae
Aflask is filled with pcl5 to a pressure of 2.00 atm at 300°c and allowed to come to equilibrium according to the reaction: pcl5(g) ⇄ pcl3(g) + cl2(g) analysis shows the total pressure in the flask at equilibrium is 3.96 atm. calculate the equilibrium constant kp for the reaction.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 05:00
Choose all the answers that apply. ionic compounds dissolve easily in water do not dissolve in water have low melting points have high melting points conduct electricity when melted
Answers: 1

Chemistry, 22.06.2019 21:00
How many neutrons does an element have if its atomic number is 50 and its mass number is 166
Answers: 1

Chemistry, 23.06.2019 06:00
What volume of 0.500 mol/l hydrochloric acid, hci (aq) is required to react completely with 1.00 g of aluminum hydroxide, ai(oh)3 (s)?
Answers: 1

Chemistry, 23.06.2019 13:00
Me puede ayudar con estas preguntas 1. diga que estudia la química orgánica explica el nacimiento bioquímicas 2. cual es la importancia de la química orgánica. 3. determine las principales características del hidrógeno, oxigeno, nitrógeno y azufre como elementos que constituyen los compuestos orgánicas. 4. elabore una tabla comparativa entre compuestas orgánicas e incaicos.
Answers: 1
You know the right answer?
Aflask is filled with pcl5 to a pressure of 2.00 atm at 300°c and allowed to come to equilibrium acc...
Questions

Mathematics, 16.10.2019 16:40





Health, 16.10.2019 16:40


Chemistry, 16.10.2019 16:40




English, 16.10.2019 16:40


Social Studies, 16.10.2019 16:40


History, 16.10.2019 16:40

Mathematics, 16.10.2019 16:40


Chemistry, 16.10.2019 16:40

History, 16.10.2019 16:40

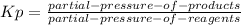
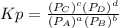
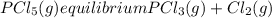
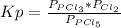
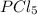 so the total pressure for the system is also the partial pressure for
so the total pressure for the system is also the partial pressure for 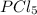 ,
,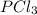 and
and 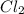 so now we need to estimate the partial pressure for each specie.
so now we need to estimate the partial pressure for each specie. 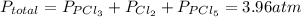
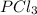 and
and 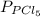 in the equilibrium will be
in the equilibrium will be