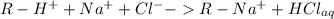Chemistry, 10.10.2019 05:30 javontiye226
Acolumn is packed with 0.5 g of a strongly acidic ion exchange resin in h+ form. a 1.0 m nacl solution is passed through the column until the eluant coming out becomes neutral. the collected eluant is completely neutralized by 17 ml. of 0.5 m naoh. the ion exchange capacity of the resin is:
(a) 1.00 meq/g(b) 1.25 meq/g
(c) 1.50 meq/g(d) 1.75 meq/g

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 17:10
There are 6.022 x 10^23 atoms of hg in 1 mole of hg. the number of atoms in 4.5 moles of hg can be found by multiplying 4.5 by 6.022 x 10^23 a. 2.7 x 10^24 b. 27 x 10^23 c. 2.71 x10^24 d. 27.099 x 10^23
Answers: 3

Chemistry, 22.06.2019 23:00
In the reaction h2co3 (aq) + 3nh3 (aq) = 2 nh4+ (aq) + co3 2-, how many electrons are transferred?
Answers: 3

Chemistry, 23.06.2019 00:40
To prevent the presence of air, noble gases are placed over highly reactive chemicals to act as inert "blanketing" gases. a chemical engineer places a mixture of noble gases consisting of 4.37 g of he, 13.36 g of ne, and 36.65 g of kr in a piston-cylinder assembly at stp. calculate the partial pressure in torr of kr.
Answers: 1

Chemistry, 23.06.2019 04:31
One student said that the investigation was not valid (a fair test). write a plan for the investigation that includes improvements to the method and apparatus
Answers: 1
You know the right answer?
Acolumn is packed with 0.5 g of a strongly acidic ion exchange resin in h+ form. a 1.0 m nacl soluti...
Questions


Mathematics, 08.10.2019 13:30

Mathematics, 08.10.2019 13:30








English, 08.10.2019 13:30


Mathematics, 08.10.2019 13:30

English, 08.10.2019 13:30

Biology, 08.10.2019 13:30

Mathematics, 08.10.2019 13:30

Physics, 08.10.2019 13:30



Mathematics, 08.10.2019 13:30