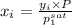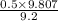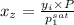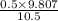Chemistry, 10.10.2019 05:30 aleahnew36
Aclosed system contains an equimolar mixture of n-pentane and isopentane. suppose the system is initially all liquid at 120°c and a high pressure, and the pressure is gradually reduced at a constant temperature. estimate the pressures at which the first bubble of vapor forms and at which the last drop of liquid evaporates. also calculate the liquid and vapor compositions (mole fractions) at those two conditions.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 17:30
How many grams of magnesium metal will react completely with 8.3 liters of 5.5 m hcl? show all of the work needed to solve this problem. mg (s) + 2hcl (aq) → mgcl2 (aq) + h2 (g)
Answers: 3

Chemistry, 22.06.2019 16:00
As changes in energy levels of electrons increase, the frequencies of atomic line spectra they emit
Answers: 2

Chemistry, 22.06.2019 18:30
Which of the following nuclei would be the least stable a 2 protons, 2 neutrons b 1 proton 1 neutron c 1 proton 3 neutrons d 1 proton 2 neutrons
Answers: 3

Chemistry, 22.06.2019 19:00
Which is the solubility product expression for caf2(s)?  [ca2+]/[f–]2  [ca2+][f2–]  [ca]+[f]2  [ca2+][f–]2
Answers: 3
You know the right answer?
Aclosed system contains an equimolar mixture of n-pentane and isopentane. suppose the system is init...
Questions


Mathematics, 11.03.2021 20:10


Mathematics, 11.03.2021 20:10

Mathematics, 11.03.2021 20:10

History, 11.03.2021 20:10

History, 11.03.2021 20:10

Mathematics, 11.03.2021 20:10



Mathematics, 11.03.2021 20:10

English, 11.03.2021 20:10




Mathematics, 11.03.2021 20:10

Mathematics, 11.03.2021 20:10

Mathematics, 11.03.2021 20:10


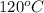 = (120 + 273.15)K = 393.15 K,
= (120 + 273.15)K = 393.15 K, 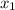 = 0.5 and
= 0.5 and 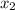 = 0.5
= 0.5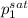 (393.15 K) = 9.2 bar
(393.15 K) = 9.2 bar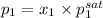
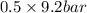
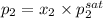
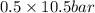
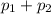
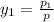
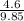
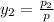
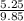
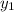 = 0.5,
= 0.5, 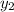 = 0.5
= 0.5 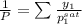
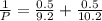
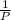 = 0.101966
= 0.101966 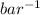
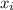 and its formula is as follows.
and its formula is as follows.