In the following experiment, a coffee-cup calorimeter containing 100 g of h2o is used. the initial temperature of the calorimeter is 23.0 oc. if 3.3 g of cacl2is added to the calorimeter, what will be the final temperature of the solution in the calorimeter? assume that the heat capacity of the solution is 4.18 j/goc, and that the heat capacity of the calorimeter is negligible. the heat of dissolution δhsoln of cacl2 is −82.8 kj/mol.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 03:00
Zoe is investigating the composition of substance a, an unknown substance. using chemical processes, she analyzes substance a and determines it is composed of sodium, oxygen, and hydrogen atoms in a ratio of 1 : 1 : 1. what is substance a? a. a compound b. an element c. a heterogeneous mixture d. a homogeneous mixture
Answers: 1

Chemistry, 22.06.2019 08:30
Analyze how limestone is weathered and identify the features that are formed as a result of this dissolution
Answers: 1

Chemistry, 22.06.2019 13:30
Why does asexual reproduction result in offspring with identicle genetic variation
Answers: 2

Chemistry, 22.06.2019 19:30
What is the common name for the compound shown here? enter the common name of the compound shown?
Answers: 2
You know the right answer?
In the following experiment, a coffee-cup calorimeter containing 100 g of h2o is used. the initial t...
Questions

Computers and Technology, 24.06.2019 01:30









Mathematics, 24.06.2019 01:30



English, 24.06.2019 01:30





English, 24.06.2019 01:30


Mathematics, 24.06.2019 01:30

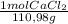 ×82800J/mol = 2462,1 J
×82800J/mol = 2462,1 J