
Chemistry, 10.10.2019 05:30 Queenbee2304
Calculate the radius of tantalum (ta) atom, given that ta has a bcc crystal structure, a density of 16.6 g/cm, and an atomic weight of 180.9 g/mol. (avogadro number, 6.023 x 103 atoms/mol).

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 03:50
Consider the reaction: n2(g) + o2(g) ? 2no(g) kc = 0.10 at 2000oc starting with initial concentrations of 0.040 mol/l of n2 and 0.040 mol/l of o2, calculate the equilibrium concentration of no in mol/l how would this be done?
Answers: 3


Chemistry, 22.06.2019 08:00
This classification of drug typically changes the brain's chemistry and reduces its ability to create its own endorphins.
Answers: 1

Chemistry, 22.06.2019 12:20
Which is an example of the practical pursuit of alchemy? a. forming perfect substances. b. transforming base metals. c. developing metalworking techniques. d. linking spiritual characteristics with material substances.
Answers: 1
You know the right answer?
Calculate the radius of tantalum (ta) atom, given that ta has a bcc crystal structure, a density of...
Questions

Biology, 17.12.2020 22:20

Physics, 17.12.2020 22:20

Chemistry, 17.12.2020 22:20

English, 17.12.2020 22:20



Chemistry, 17.12.2020 22:20

Mathematics, 17.12.2020 22:20

English, 17.12.2020 22:20

Mathematics, 17.12.2020 22:20

Geography, 17.12.2020 22:20

Mathematics, 17.12.2020 22:20

Mathematics, 17.12.2020 22:20




Mathematics, 17.12.2020 22:20



Biology, 17.12.2020 22:20

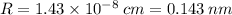
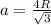
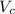 is
is 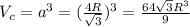

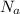 = Avogadro’s number (
= Avogadro’s number (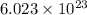 atoms/mol)
atoms/mol)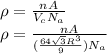
![\rho=\frac{nA}{(\frac{64\sqrt{3}R^3}{9})N_{a}}\\\frac{64\sqrt{3}R^3}{9}=\frac{nA}{\rho N_{a}}\\R^3=\frac{nA}{\rho N_{a}}\cdot \frac{1}{\frac{64\sqrt{3}}{9}} \\R=\sqrt[3]{\frac{nA}{\rho N_{a}}\cdot \frac{1}{\frac{64\sqrt{3}}{9}}}](/tpl/images/0306/0375/e96f7.png)
![R=\sqrt[3]{\frac{2\cdot 180.9}{16.6\cdot 6.023 \times 10^{23}}\cdot \frac{1}{\frac{64\sqrt{3}}{9}}}\\R = 1.43 \times 10^{-8} \:cm = 0.143 \:nm](/tpl/images/0306/0375/377b1.png)


