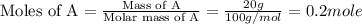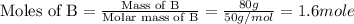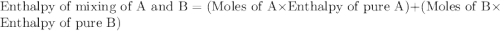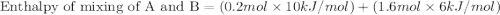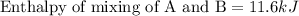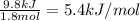Chemistry, 10.10.2019 06:00 cheerleaderautumnche
A20 wt% a solution is obtained by mixing a component with b in an insulated mixer at steady state. for every mole of solution 1 kj is removed to keep the system temperature constant. determine the enthalpy of mixture for this solution. molecular weight of a: 100 g/mol; molar enthalpy of pure a: 10 kj/mol molecular weight of b: 50 g/mol; molar enthalpy of pure b: 6 kj/mol

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 12:00
Ican determine the molar mass of an element by looking on the under the atomic mass for the element. for example the molar mass of phosphorus is 30.974 grams/mole. avogadro’s number tells me the amount of representative particles in 1 mole of any substance. this means 12.011 gram sample of carbon and a 32.0 gram sample of sulfur have the same number of atoms.
Answers: 1

Chemistry, 22.06.2019 18:00
Hydrogenation reactions, in which h2 and an "unsaturated" organic compound combine, are used in the food, fuel, and polymer industries. in the simplest case, ethene (c2h4) and h2 form ethane (c2h6). if 140 kj is given off per mole of c2h4 reacting, how much heat (in mj) is released when 12 kg of c2h6 forms?
Answers: 2


Chemistry, 23.06.2019 03:50
Which best describes the activation energy of a chemical reaction? a. the combined energy of all the reactants b. the amount of energy required for a reaction to occur c. the difference in energy between products and reactants d. the potential energy stored in the bonds of reactants and products
Answers: 1
You know the right answer?
A20 wt% a solution is obtained by mixing a component with b in an insulated mixer at steady state. f...
Questions


Computers and Technology, 25.02.2022 19:30












Chemistry, 25.02.2022 19:30

Geography, 25.02.2022 19:30

Biology, 25.02.2022 19:30

History, 25.02.2022 19:30



Social Studies, 25.02.2022 19:30