
Chemistry, 10.10.2019 05:00 akaeiraspruell
Use the following equilibrium reaction and constant for the deprotonation of bicarbonate (hco3-) to carbonate (co32-) to determine: hco3 = co2 + h+ k = 10-10.33 (a) whether hco3 or co32- would dominate at ph 9.1 and (b) what the concentration of [co32-] would be at this ph if [hco3 ] = 10-6 m

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 18:00
When the following equation is balanced using the smallest possible integers, what is the coefficent of oxygen gas? c7h16o(g) + o2(g) → co2(g) + h2o(g) -1 -5 -8 -16 -21
Answers: 3

Chemistry, 21.06.2019 22:00
What is driving behind plate tectonics (plate movment)? a) gravity only b) inertia c) convection and gravity d) the sun theres no option for science so i picked chemistry. plz
Answers: 2

Chemistry, 22.06.2019 01:00
Consider three unlabeled bottles, each contain small pieces of one of the following metals. - magnesium - sodium - silver the following reagents are used for identifying the metals. - pure water - a solution of 1.0 molar hcl - a solution of concentrated hno3 (a) which metal can be easily identified because it is much softer than the other two? describe a chemical test that distinguishes this metal from the other two, using only one of the reagents above. write a balanced chemical equation for the reaction that occurs. (b) one of the other two metals reacts readily with the hcl solution. identify the metal and write the balanced chemical equation for the reaction that occurs when this metal is added to the hcl solution. use the table of standard reduction potentials (attached) to account for the fact that this metal reacts with hcl while the other does not. (c) the one remaining metal reacts with the concentrated hno3 solution. write a balanced chemical equation for the reaction that occurs. (d) the solution obtained in (c) is diluted and a few drops of 1 m hcl is added. describe what would be observed. write a balanced chemical equation for the reaction that occurs.
Answers: 2

Chemistry, 22.06.2019 12:30
The melting point of sulfur is 115 °c and its boiling point is 445 °c. what state would sulfur be in at 200 °c?
Answers: 1
You know the right answer?
Use the following equilibrium reaction and constant for the deprotonation of bicarbonate (hco3-) to...
Questions

Chemistry, 09.02.2021 07:00


Mathematics, 09.02.2021 07:00

Chemistry, 09.02.2021 07:00

English, 09.02.2021 07:00

Mathematics, 09.02.2021 07:00


English, 09.02.2021 07:00

Mathematics, 09.02.2021 07:00



Geography, 09.02.2021 07:00



Physics, 09.02.2021 07:00

Computers and Technology, 09.02.2021 07:00

Mathematics, 09.02.2021 07:00

History, 09.02.2021 07:00


Mathematics, 09.02.2021 07:00

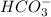 will dominate at pH = 9.1
will dominate at pH = 9.1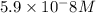
![pH=-\log[H^+]](/tpl/images/0305/9437/cf945.png) ......(1)
......(1)![9.1=-\log[H^+]](/tpl/images/0305/9437/e5ddb.png)
![[H^+]=10^{-9.1}](/tpl/images/0305/9437/b7f9d.png)
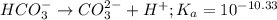
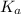 for above reaction follows:
for above reaction follows:![K_a=\frac{[CO_3^{2-}]\times [H^+]}{[HCO_3^-]}](/tpl/images/0305/9437/fea1b.png)
![10^{-10.33}=\frac{[CO_3^{2-}]\times 10^{-9.1}}{[HCO_3^-]}\\\\\frac{[HCO_3^-]}{[CO_3^{2-}]}=\frac{10^{-9.1}}{10^{-10.33}}\\\\\frac{[HCO_3^-]}{[CO_3^{2-}]}=16.98](/tpl/images/0305/9437/786f2.png)
![[HCO_3^-]=16.98\times [CO_3^{2-}]](/tpl/images/0305/9437/e6ce5.png)
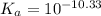
![[HCO_3^-]=10^{-6}M](/tpl/images/0305/9437/1335d.png)
![10^{-10.33}=\frac{[CO_3^{2-}]\times 10^{-9.1}}{10^{-6}}](/tpl/images/0305/9437/27401.png)
![[CO_3^{2-}]=\frac{10^{-6}\times 10^{-10.33}}{10^{-9.1}}=5.9\times 10^-8}M](/tpl/images/0305/9437/65347.png)


