
Chemistry, 10.10.2019 03:30 Imamdiallo18
Consider the reaction 2 x2y + z2 ⇌ 2 x2yz which has a rate law of rate= k[x2y][z2] select a possible mechanism for the reaction. group of answer choices
step 1: z2 --> z + z (slow) step 2: x2y + z → x2yz (fast) step 3: x2y + z → x2yz (fast) step 1: x2y + z2→ x2yz2 (slow) step 2: x2yz2 → x2yz + z (fast) step 1: 2 x2y + z2 → 2x2yz (slow) step 1: x2y + z2 → x2yz + z (slow) step 2: x2y + z → x2yz (fast) step 1: 2 x2y ⇌ x4y2 (fast) step 2: x4y2 + z2 → 2 x2yz (slow)

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 11:50
Acompound has a molecular weight of 12.124 atomic mass units and the empirical formula c3h40. what is the molecular formula of the compound?
Answers: 3

Chemistry, 22.06.2019 13:50
Amap that uses a range of colors and shading to represent the elevation, depth, or landscape of specific features on earth is a/an map.
Answers: 3

Chemistry, 22.06.2019 14:00
How does the presence of oxygen affect the chemical pathways used to extract energy from glucose?
Answers: 3

Chemistry, 23.06.2019 05:40
Which order shows the levels of organization from largest to smallest? organism, organ system, cell, organ, tissue organism, tissue, organ system, organ, cell organism, organ, organ system, cell, tissue organism, organ system, organ, tissue, cell
Answers: 2
You know the right answer?
Consider the reaction 2 x2y + z2 ⇌ 2 x2yz which has a rate law of rate= k[x2y][z2] select a possible...
Questions


Mathematics, 07.01.2020 00:31





Mathematics, 07.01.2020 00:31


Chemistry, 07.01.2020 00:31

English, 07.01.2020 00:31


Social Studies, 07.01.2020 00:31

Mathematics, 07.01.2020 00:31

Social Studies, 07.01.2020 00:31


Mathematics, 07.01.2020 00:31


Mathematics, 07.01.2020 00:31

History, 07.01.2020 00:31

Mathematics, 07.01.2020 00:31

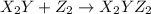 (slow)
(slow)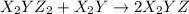 (fast)
(fast)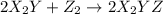
![Rate=k[X_2Y][Z_2]](/tpl/images/0305/6835/e6034.png)
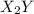 and
and 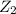 .
.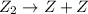 (slow)
(slow)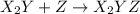 (fast)
(fast)![Rate=K[Z_2]](/tpl/images/0305/6835/c66c5.png)
![Rate=k[X_2Y]^2[Z_2]](/tpl/images/0305/6835/8a2d6.png)
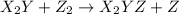 (fast)
(fast)![Rate=K'[X_2Y][Z]](/tpl/images/0305/6835/3c7d4.png) .............(1)
.............(1)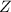 , we get:
, we get:![\frac{d[Z]}{dt}=K"[X_2Y][Z_2]](/tpl/images/0305/6835/87d2e.png) .........(2)
.........(2)![Rate=K'K"[X_2Y]^2[Z_2]](/tpl/images/0305/6835/e4aa1.png)
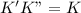
![Rate=K[X_2Y]^2[Z_2]](/tpl/images/0305/6835/5d2a6.png)
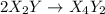 (fast)
(fast)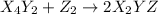 (slow)
(slow)![Rate=K'[X_4Y_2][Z_2]](/tpl/images/0305/6835/57b59.png) .............(1)
.............(1)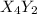 , we get:
, we get:![\frac{d[X_4Y_2]}{dt}=K"[X_2Y]^2](/tpl/images/0305/6835/bce7e.png) .........(2)
.........(2)

