
In the lab you measure a clean dry crucible and cover to be 24.36 grams. you obtain a 2cm piece of pure magnesium metal. after sand papering the magnesium you place it in into your crucible and the mass of crucible, cover and magnesium masses out to be 24.66 grams. you observe that the magnesium is gray and shiny. you heat the crucible gently at first then vigorous for 10 minutes until the magnesium ignites. after the crucible cools you add a few drops of distilled water and heat again. the mass of the magnesium compound, crucible, and cover masses out to be 24.85 grams.
what is the empirical formula of the compound? show all work and units.
1) mass of the mg
2) mass of the mgo
3) mass of 0
4) moles of mg & moles of o
5) show mole to mole mgxoy
6) empricial formula of compound

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 12:00
Ageochemist examines a piece of metal that he found in the soil. he performs tests to identify the metal from its density, electrical conductivity, and melting point. which statement best describes his investigation? a. he is determining physical properties that are sufficient to identify the metal.b. he is determining chemical properties that are sufficient to identify the metal.c. he is determining physical properties that are insufficient to identify the metal.d. he is determining chemical properties that are insufficient to identify the metal.
Answers: 3

Chemistry, 23.06.2019 14:00
How is the electron sea model of metallic bonding different from the band theory? how are they the same? give at least one similarity and one difference between the models
Answers: 2

Chemistry, 23.06.2019 14:00
Cassandra made a venn diagram to compare and contrast the two stages of cellular respiration. which belongs in the area marked x? energy is released. oxygen is used up. glucose is broken down. carbon dioxide is used up.
Answers: 1

Chemistry, 23.06.2019 14:00
Fill in the blanks for the following statements: the rms speed of the molecules in a sample of h2 gas at 300 k will be times larger than the rms speed of o2 molecules at the same temperature, and the ratio µrms (h2) / µrms (o2) with increasing temperature.
Answers: 1
You know the right answer?
In the lab you measure a clean dry crucible and cover to be 24.36 grams. you obtain a 2cm piece of p...
Questions

English, 27.09.2020 18:01











Mathematics, 27.09.2020 18:01

Mathematics, 27.09.2020 18:01



Mathematics, 27.09.2020 18:01

Mathematics, 27.09.2020 18:01


Mathematics, 27.09.2020 18:01

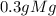 *
*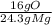 = 0.2g O
= 0.2g O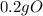 *
*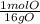 = 0.01mol O
= 0.01mol O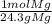 = 0.01mol Mg
= 0.01mol Mg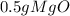 *
*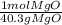 = 0.01mol MgO
= 0.01mol MgO

