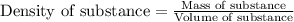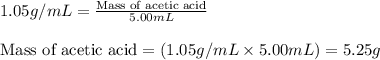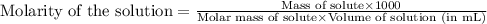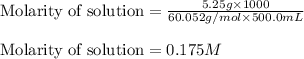"pure acetic acid (hc2h3o2) is a liquid and is known as glacial acetic acid. calculate the molarity of a solution prepared by dissolving 5.00 ml of glacial acetic acid at 25 °c in sufficient water to give 500.0 ml of solution. the density of glacial acetic acid at 25 °c is 1.05 g/ml. pure acetic acid (hc2h3o2) is a liquid and is known as glacial acetic acid. calculate the molarity of a solution prepared by dissolving 5.00 ml of glacial acetic acid at 25 °c in sufficient water to give 500.0 ml of solution. the density of glacial acetic acid at 25 °c is 1.05 g/ml.
a. 3.50 × 10-5 m
b. 126 m
c. 0.0350 m
d. 2.10 m
e. 2.10 × 10-3 m

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 07:00
Which atom or ion is the largest? a. k b. k+ c. ca d. ca2+ e. li
Answers: 1

Chemistry, 23.06.2019 10:30
When a chemist collects hydrogen gas over water, she ends up with a mixture of hydrogen and water vapor in her collecting bottle if the pressure in the collecting bottle is 97.1 kilopascals and the vapor pressure of the water is 3 2 kilopascals, what is the partial pressure of the hydrogen?
Answers: 1

Chemistry, 23.06.2019 11:00
Which example is a mechanical wave? a.microwave b.radio wave c.water wave d.ultraviolet light
Answers: 1

Chemistry, 23.06.2019 13:30
What is matter? a. anything that has mass and takes up space b. something that has volume and takes up space. c. things that have energy and take up space d. things that take up space but don't have mass
Answers: 2
You know the right answer?
"pure acetic acid (hc2h3o2) is a liquid and is known as glacial acetic acid. calculate the molarity...
Questions


History, 25.06.2019 22:20





History, 25.06.2019 22:20

Mathematics, 25.06.2019 22:20

Mathematics, 25.06.2019 22:20


Mathematics, 25.06.2019 22:20


Social Studies, 25.06.2019 22:20



Mathematics, 25.06.2019 22:20