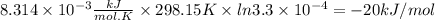Chemistry, 10.10.2019 00:30 idontknow1993
Acetylsalicylic acid (hc9h7o4) is a weak acid that dissociates in water in the following manner. hc9h7o4(aq) + h2o(l) equilibrium reaction arrow c9h7o4−(aq) + h3o+(aq) at a temperature of 298.15 k, the acid-dissociation constant (ka) for acetylsalicylic acid is 3.3 ✕ 10−4. (a) calculate the change in standard free energy (δg°) for this equilibrium reaction. kj/mol (b) what is the value of δg at equilibrium? δg > 0 δg = 0 δg < 0 (c) if the reaction were spontaneous in the forward direction, what type of value would you expect for δg?

Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 20:30
What is a difference between a mixture of elements and a mixture of compounds
Answers: 1

Chemistry, 22.06.2019 22:00
The diagrams to the right show the distribution and arrangement of gas particles in two different containers. according to kinetic-molecular theory, which of the following statements is true? check all that apply. if the temperatures of both containers are equal, container a has greater pressure than container b. if the volume of container a decreased, its pressure would decrease. if the pressure in both containers is equal, container a has a lower temperature than container b.
Answers: 2

Chemistry, 23.06.2019 02:20
In a chemical reaction, the final amount of the products is determined by the a. universal gas law b. law of definite proportions c. air pressure d. temperature e. none of the above me
Answers: 2
You know the right answer?
Acetylsalicylic acid (hc9h7o4) is a weak acid that dissociates in water in the following manner. hc9...
Questions

English, 26.08.2019 08:10



English, 26.08.2019 08:10

Social Studies, 26.08.2019 08:10



Mathematics, 26.08.2019 08:10







Chemistry, 26.08.2019 08:10


Mathematics, 26.08.2019 08:10

History, 26.08.2019 08:10

Social Studies, 26.08.2019 08:10