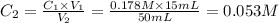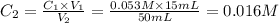Achemist places 2.5316 g of na 2so 4 in a 100 ml volumetric flask and adds water to the mark. she then pipets 15 ml of the resulting solution into a 50 ml volumetric flask and adds water to the mark and mixes to make a solution. she then pipets 15 ml of this new solution into a 50 ml volumetric flask and dilutes to the mark. determine the molar concentration of sodium sulfate in the most dilute solution prepared.

Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 02:30
Asa choose the correct set of reaction coefficients to properly balance the following chemical equation according to the law of conservation of mass: __s8 + __o2 ==> __so2 1, 1, 8 1, 8, 1 1, 8, 8 8, 1, 1
Answers: 1

Chemistry, 22.06.2019 12:40
Quiz1. which physical state of nitrogen has the highest entropy? a solid© b gasoc liquid
Answers: 1

Chemistry, 23.06.2019 00:30
Titration reveals that 11.6 ml of 3.0m sulfuric acid are required to neutralize the sodium hydroxide in 25.00ml of naoh solution. what is the molarity of the naoh solution?
Answers: 1
You know the right answer?
Achemist places 2.5316 g of na 2so 4 in a 100 ml volumetric flask and adds water to the mark. she th...
Questions

Mathematics, 28.04.2021 21:20

Mathematics, 28.04.2021 21:20


Mathematics, 28.04.2021 21:20


Mathematics, 28.04.2021 21:20

Social Studies, 28.04.2021 21:20




Mathematics, 28.04.2021 21:20


Mathematics, 28.04.2021 21:20






Mathematics, 28.04.2021 21:20

![[Na_{2}SO_{4}]=\frac{moles(Na_{2}SO_{4})}{liters(solution)} =\frac{mass((Na_{2}SO_{4}))}{molarmass(moles(Na_{2}SO_{4}) \times 0.100L)} =\frac{2.5316g}{142g/mol\times 0.100L } =0.178M](/tpl/images/0304/7678/6942e.png)