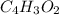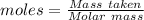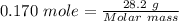Terephthalic acid is an important chemical used in the manufacture of polyesters and plasticizers. it contains only , , and . combustion of 50.20 mg terephthalic acid produces 106.4 mg and 16.34 mg . if 0.170 mole of terephthalic acid has a mass of 28.2 g, determine the molecular formula for terephthalic acid.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 18:30
In an oxidation-reduction reaction, oxidation is what happens when a reactant
Answers: 1

Chemistry, 22.06.2019 10:30
What determines the average kinetic energy of the particles in a gas? a. the number of collisions b. the number of particles c. the size of the particles d. the temperature
Answers: 1

Chemistry, 22.06.2019 14:10
Aconcentrated solution of ammonia is 14.8m and has a density of 0.899g/l. what is the concentration of ammonia in this solution in weight percent (%w/w)?
Answers: 1

Chemistry, 23.06.2019 05:00
Asolution is made by dissolving 2.3 moles of sodium chloride (nacl) in 0.155 kilograms of water. if the molal boiling point constant for water (kb) is 0.51 °c/m, what would be the boiling point of this solution? show all the steps taken to solve this problem.
Answers: 1
You know the right answer?
Terephthalic acid is an important chemical used in the manufacture of polyesters and plasticizers. i...
Questions



English, 15.04.2020 22:52

French, 15.04.2020 22:53

Spanish, 15.04.2020 22:53



Mathematics, 15.04.2020 22:53




Mathematics, 15.04.2020 22:53



Biology, 15.04.2020 22:53

English, 15.04.2020 22:53

History, 15.04.2020 22:53

Mathematics, 15.04.2020 22:53

Biology, 15.04.2020 22:53

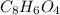
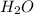 = 16.34 ×10⁻³ g /18 g/mol = 0.000907778 moles
= 16.34 ×10⁻³ g /18 g/mol = 0.000907778 moles
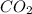 = 106.4 ×10⁻³ g /44.01 g/mol = 0.002418 moles
= 106.4 ×10⁻³ g /44.01 g/mol = 0.002418 moles