
Consider the overall reaction: 2 x2y + z2 ⇌ 2 x2yz which has an experimentally determined rate law of: rate= k[x2y][z2]. which of the following are possible mechanisms for the reaction? group of answer choices step 1: 2 x2y + z2 → 2x2yz (slow) step 1: x2y + z2 ⇌ x2yz + z (fast) step 2: x2y + z → x2yz (slow) step 1: x2y + z2→ x2yz2 (slow) step 2: x2yz2 + x2y → 2 x2yz (fast) step 1: 2 x2y ⇌ x4y2 (fast) step 2: x4y2 + z2 → 2 x2yz (slow) step 1: z2 → z + z (slow) step 2: x2y + z → x2yz (fast) step 3: x2y + z → x2yz (fast) none of the above

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 08:30
For each of the compounds below, show that the charges on the ions add up to zero. a. kbr b. cao c. li(2)o d. cacl(2) e. alcl(3)
Answers: 2


Chemistry, 22.06.2019 18:40
What is the binding energy of a nucleus that has a mass defect of 5.81*10-^29 kg a 5.23*10-^12 j b 3.15* 10^12 j c 1.57*10-3 j d 9.44*10^20 j
Answers: 1

Chemistry, 22.06.2019 20:00
What happens to the temperature of a substance when the average kinetic energy of its particles increases?
Answers: 3
You know the right answer?
Consider the overall reaction: 2 x2y + z2 ⇌ 2 x2yz which has an experimentally determined rate law...
Questions



History, 17.02.2020 03:21


Biology, 17.02.2020 03:21


Mathematics, 17.02.2020 03:22

Mathematics, 17.02.2020 03:22

Mathematics, 17.02.2020 03:22

Biology, 17.02.2020 03:22

Mathematics, 17.02.2020 03:22

Mathematics, 17.02.2020 03:22


Physics, 17.02.2020 03:22

Physics, 17.02.2020 03:23



Biology, 17.02.2020 03:24


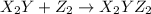 (slow)
(slow)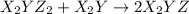 (fast)
(fast)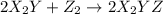
![Rate=k[X_2Y][Z_2]](/tpl/images/0304/3789/e6034.png)
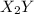 and
and 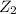 .
.![Rate=k[X_2Y]^2[Z_2]](/tpl/images/0304/3789/8a2d6.png)
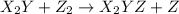 (fast)
(fast)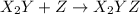 (slow)
(slow)![Rate=K'[X_2Y][Z]](/tpl/images/0304/3789/3c7d4.png) .............(1)
.............(1)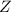 , we get:
, we get:![\frac{d[Z]}{dt}=K"[X_2Y][Z_2]](/tpl/images/0304/3789/87d2e.png) .........(2)
.........(2)![Rate=K'K"[X_2Y]^2[Z_2]](/tpl/images/0304/3789/e4aa1.png)
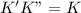
![Rate=K[X_2Y]^2[Z_2]](/tpl/images/0304/3789/5d2a6.png)
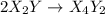 (fast)
(fast)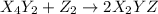 (slow)
(slow)![Rate=K'[X_4Y_2][Z_2]](/tpl/images/0304/3789/57b59.png) .............(1)
.............(1)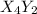 , we get:
, we get:![\frac{d[X_4Y_2]}{dt}=K"[X_2Y]^2](/tpl/images/0304/3789/bce7e.png) .........(2)
.........(2)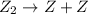 (slow)
(slow)![Rate=K[Z_2]](/tpl/images/0304/3789/c66c5.png)


