
Chemistry, 09.10.2019 19:10 ohartshorn1599
Heavy metal ions like lead(ii) can be precipitated from laboratory wastewater by adding sodium sulfide, na2s. will all the lead be removed from 11.2 ml of 7.10×10-3 m pb(no3)2 upon addition of 12.4 ml of 0.0117 m na2s? if all the lead is removed, how many moles of lead is this? if not, how many moles of pb remain?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 01:20
Match the acid base pairs by arranging the acid name with the conjugate base formula. hydrogen carbonate hydrogen phosphate carbonic acid read water sulfuric acid phosphoric acid a. co32- b. hso4- c. hco3- d. po43- e. h2po4- f. oh-
Answers: 1

Chemistry, 22.06.2019 01:30
The table lists pressure and volume values for a particular gas. which is the best estimate for the value of v at p = 7.0 × 103 pascals?
Answers: 3

Chemistry, 22.06.2019 05:30
According to periodic trend, which of the following most likely has the highest ionization energy? kr be ni sc
Answers: 3

Chemistry, 22.06.2019 07:30
According to the vsepr theory what is the shape of a molecule that has a central atom valence three other items with no lone pairs of electrons
Answers: 1
You know the right answer?
Heavy metal ions like lead(ii) can be precipitated from laboratory wastewater by adding sodium sulfi...
Questions



Medicine, 09.01.2020 04:31

Physics, 09.01.2020 04:31

Mathematics, 09.01.2020 04:31



Physics, 09.01.2020 04:31




Medicine, 09.01.2020 04:31

Medicine, 09.01.2020 04:31





Medicine, 09.01.2020 04:31

Medicine, 09.01.2020 04:31

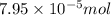
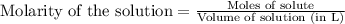
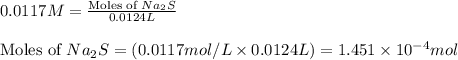
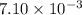 M
M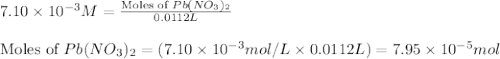
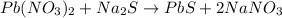
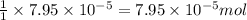 of sodium sulfide.
of sodium sulfide.

