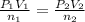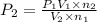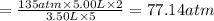Chemistry, 09.10.2019 18:00 carolelai08
Imagine that you have a 5.00 l gas tank and a 3.50 l gas tank. you need to fill one tank with oxygen and the other with acetylene to use in conjunction with your welding torch. if you fill the larger tank with oxygen to a pressure of 135 atm , to what pressure should you fill the acetylene tank to ensure that you run out of each gas at the same time? assume ideal behavior for all gases. express your answer with the appropriate units. view available hint(s) pp = nothingnothing submit provide feedback next

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 17:30
Which term describes a fracture in the earth at which land stays in the same place? a. joint b. fault c. split d. hinge
Answers: 1

Chemistry, 22.06.2019 06:30
Identify the missing numbers below to show the result of multiplying the numbers (1.6 × 10-19)(5.0 × 106) = c × 10d
Answers: 1

Chemistry, 22.06.2019 10:50
An atom of lithium-7 has an equal number of(1) electrons and neutrons(2) electrons and protons(3) positrons and neutrons(4) positrons and protons
Answers: 2

Chemistry, 22.06.2019 12:30
Suppose you wanted to make 100 grams of water. what is the molar mass of water (h2o)?
Answers: 2
You know the right answer?
Imagine that you have a 5.00 l gas tank and a 3.50 l gas tank. you need to fill one tank with oxygen...
Questions

Health, 14.07.2019 20:30


History, 14.07.2019 20:30

Mathematics, 14.07.2019 20:30



Mathematics, 14.07.2019 20:30

History, 14.07.2019 20:30


English, 14.07.2019 20:30

Mathematics, 14.07.2019 20:30

English, 14.07.2019 20:30

Mathematics, 14.07.2019 20:30



Mathematics, 14.07.2019 20:30


Mathematics, 14.07.2019 20:30

Mathematics, 14.07.2019 20:30

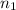
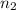
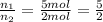
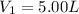
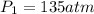
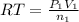 ..[1]
..[1]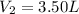
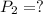
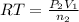 ..[2]
..[2]