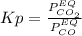Chemistry, 09.10.2019 05:30 minnahelhoor
Nickel (ii) oxide reacts with carbon monoxide to form nickel metal. co(g) + nio(s) ⇄ co2(g) + ni(s) kp = 20 at 873 k if a reaction vessel at equilibrium contains solid ni, solid nio, 400 mm hg of co2, and 20 mm hg of co, doubling the amount of co(g) present will lead to the production of more solid nickel at 873 k. group of answer choices

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 23:00
The wave shown on the electromagnetic spectrum disturb the medium it passes through a)different frequency. b)the same frequency .
Answers: 2

Chemistry, 22.06.2019 03:30
In saturated organic compounds, all the bonds between carbon atoms are called?
Answers: 1

Chemistry, 22.06.2019 19:00
What is the compound name for the formula [ru(en)2cl2]2+ and [co(en)cl2br]-
Answers: 1

Chemistry, 22.06.2019 22:00
Scientists often have to deal with numbers that are either very large or very small. for example, the radius of the sun is approximately 696,000 kilometers, while bacterial cells are as small as 1.9 × 10-4 millimeters. express each number in an alternate form.
Answers: 1
You know the right answer?
Nickel (ii) oxide reacts with carbon monoxide to form nickel metal. co(g) + nio(s) ⇄ co2(g) + ni(s)...
Questions


History, 18.03.2021 03:20

Biology, 18.03.2021 03:20

Mathematics, 18.03.2021 03:20

Mathematics, 18.03.2021 03:20

Mathematics, 18.03.2021 03:20





English, 18.03.2021 03:20

History, 18.03.2021 03:20




History, 18.03.2021 03:20

Mathematics, 18.03.2021 03:20

Mathematics, 18.03.2021 03:20