
Consider a mixture of air and gasoline vapor in a cylinder with a piston. the original volume is 42 cm3. if the combustion of this mixture releases 927 j of energy, to what volume (in l) will the gases expand against a constant pressure of 656 torr if all the energy of combustion is converted into work to push back the piston?

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 14:40
What type of solution is formed if 10 g of kclo3 are dissolved in 100g of water at 30
Answers: 2

Chemistry, 21.06.2019 17:00
Look at the reaction below: ca(hco3)2 --> caco3 + co2 + h2o first, balance the reaction. once balanced, use dimensional analysis or another method to find out how many moles of carbon dioxide will be produced if we start with 16.5 moles of calcium bicarbonate (calcium hydrogen carbonate). = mol of co2 number needs to be reported to three significant figures.
Answers: 1


Chemistry, 22.06.2019 05:30
Astudent carefully transfers 30 g of water and 30 g of alcohol in a glass tube, forming two layers and filling the tube completely. after sealing the tube, the student mixes the solutions, and notices a bubble that forms in the tube. what is the mass of the contents in the glass tube after mixing?
Answers: 2
You know the right answer?
Consider a mixture of air and gasoline vapor in a cylinder with a piston. the original volume is 42...
Questions


Arts, 25.02.2021 22:10

Mathematics, 25.02.2021 22:10

Mathematics, 25.02.2021 22:10


Mathematics, 25.02.2021 22:10


Mathematics, 25.02.2021 22:10

History, 25.02.2021 22:10

Chemistry, 25.02.2021 22:10


Mathematics, 25.02.2021 22:10




Mathematics, 25.02.2021 22:10


Spanish, 25.02.2021 22:10

Mathematics, 25.02.2021 22:10

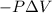
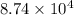 Pa (as 1 torr = 133.3 Pa)
Pa (as 1 torr = 133.3 Pa)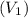 = 42
= 42 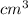
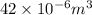 (as 1 m = 100 cm)
(as 1 m = 100 cm)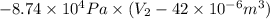
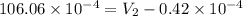
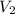 =
= 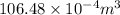
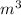 = 1000 L)
= 1000 L)

