
Chemistry, 09.10.2019 03:30 cranfordjacori
An aqueous solution of sodium sulfate is allowed to react with an aqueous solution of calciumnitrate. the complete ionic equation contains which of the following species (when balanced in stan-dard form)? a.2na+aq)b.2so42-(aq)c.3ca2+(aq)d. no3-(aq)e. k+(aq)

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 11:50
Which of the following statements about hybrid orbitals is or are true? choose all that apply. choose all that apply. under sp2 hybridization, the large lobes point to the vertices of an equilateral triangle. after an atom undergoes sp hybridization there is one unhybridized p orbital on the atom. the angle between the large lobes of sp3 hybrids is 109.5∘
Answers: 2

Chemistry, 22.06.2019 14:30
Which of the following describes a situation where competition between producers exists
Answers: 1


Chemistry, 23.06.2019 00:30
Ok, so i have 2 questions. try to answer them both: (the topic is fire) 1) how can you represent the chemical reaction of fire? 2) what kind of bond is formed in this chemical reaction
Answers: 3
You know the right answer?
An aqueous solution of sodium sulfate is allowed to react with an aqueous solution of calciumnitrate...
Questions

History, 20.11.2020 01:00

Advanced Placement (AP), 20.11.2020 01:00






Social Studies, 20.11.2020 01:00

English, 20.11.2020 01:00

Geography, 20.11.2020 01:00




Mathematics, 20.11.2020 01:00

Mathematics, 20.11.2020 01:00

Mathematics, 20.11.2020 01:00

Mathematics, 20.11.2020 01:00

Computers and Technology, 20.11.2020 01:00

Mathematics, 20.11.2020 01:00

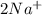
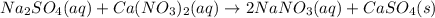
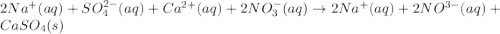
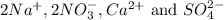 are the spectator ions.
are the spectator ions.

