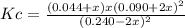Chemistry, 09.10.2019 03:00 hi510hello
Consider 2 nocl(g) \longleftrightarrow⟺ 2 no(g) + cl2 (g) at 25oc under conditions other than equilibrium, there are 1.20 moles of nocl , 0.450 moles of no, and 0.220 moles of cl2 in a 5.00 l flask. kc = 1.86 x 10-1. show work for credit. a. calculate q. b. predict the direction of the reaction. c. calculate equilibrium concentrations of all species present. the equilibrium concentration of cl2 is 0.0490 m.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 16:00
Endeleev saw trends in the physical and chemical properties of elements when he organized them by
Answers: 2

Chemistry, 22.06.2019 04:00
Write the empirical chemical formula of calcium with a mass percent of 38.8, phosphorus with a mass percent of 20.0, and oxygen with a mass percent of 41.3.
Answers: 1


You know the right answer?
Consider 2 nocl(g) \longleftrightarrow⟺ 2 no(g) + cl2 (g) at 25oc under conditions other than equili...
Questions

Mathematics, 18.07.2019 13:00




Mathematics, 18.07.2019 13:00


Mathematics, 18.07.2019 13:00




History, 18.07.2019 13:00






History, 18.07.2019 13:00


Mathematics, 18.07.2019 13:00

![Q = \frac{[C]^cx[D]^d}{[A]^ax[B]^b}](/tpl/images/0302/3128/03e8d.png)
![Q = \frac{[Cl2]x[[NO]^2}{[NOCl]^2}](/tpl/images/0302/3128/de159.png)