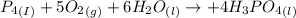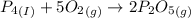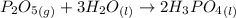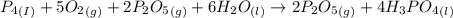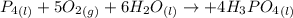Phosphoric acid, which is commonly used as rust inhibitor, food additive and etching agent for dental and orthopedic use, can be synthesized using a two-step thermal process. in the first step, phosphorus and oxygen react to form diphosphorus pentoxide: (l)(g)(g) in the second step, diphosphorus pentoxide and water react to form phosphoric acid: (g)(l)(l) write the net chemical equation for the production of phosphoric acid from phosphorus, oxygen and water. be sure your equation is balanced.

Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 13:00
In a copper wire, a temperature increase is the result of which of the following
Answers: 1

Chemistry, 22.06.2019 17:30
Aroller coaster is traveling at 13 mi./s when you purchase a hill that is 400 m long and down the hill exonerate at 4.0 m/s squared what is the final velocity of the posterior found your answer to the nearest number
Answers: 1

Chemistry, 22.06.2019 22:00
8) warming your hands by a fire is an example if which heat transfer? a. conduction b. convection c. radiation d. none of these
Answers: 1
You know the right answer?
Phosphoric acid, which is commonly used as rust inhibitor, food additive and etching agent for denta...
Questions

English, 16.12.2020 04:00


Business, 16.12.2020 04:00



Mathematics, 16.12.2020 04:00



Computers and Technology, 16.12.2020 04:00

Biology, 16.12.2020 04:00


Mathematics, 16.12.2020 04:00


Mathematics, 16.12.2020 04:00


Mathematics, 16.12.2020 04:00