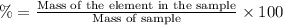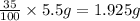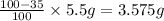Chemistry, 09.10.2019 01:00 maltipoosarecool
A5.5 g sample of a substance contains only carbon and oxygen. carbon makes up 35% of the mass of the substance. the rest is made of oxygen. you are asked to determine the mass of oxygen in the sample. which of the following expressions demonstrates a mathematical procedure to solve this problem using the proper order of operations? 1) ((100 - 35)/100) times 5.5 grams = 2)100 - 35/100 times 5.5 grams = 3) 100 - (35/100) times 5.5 grams = *recall that to calculate a percent of an amount in grams means you have to convert the percent to a number and multiply by the amount in grams. for example, 50% means 50 out of 100, or 50 hundredths. so 50% of 10 grams is 0.50 times 10 grams. you need to take 0.50 and multiply it by 10 grams to determine 50% of 10 grams is 5.0. (50% and 10 grams are examples and not numbers for this problem.)

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 08:30
Sally is making a model of a magnesium atom with an atomic mass number of 24 for her chemistry class. she has foam balls for the protons, neutrons, and electrons. she has added 6 neutrons to her model so far. how many more neutrons does she need to add to complete her neutral atom of magnesium?
Answers: 1

Chemistry, 22.06.2019 10:00
Americium-241 undergoes fission to produce three neutrons per fission event. if a neutron-absorbing material is mixed in with this sample so that the rate of neutron production drops down to 1.8 neutrons per fission event, which will be effective at achieving a critical mass? check all that apply. remove a deflective shield surrounding the sample. remove absorbent material mixed in with the sample. compress the sample of americium-241.
Answers: 1

Chemistry, 22.06.2019 13:00
Lab reagent, hypothesis test.a reference solution used as a lab reagent is purported to have a concentration of 5 mg/dl. six samples are taken from this solution and the following concentrations are recorded: (5.32, 4.88, 5.10, 4.73, 5.15, 4.75) mg/dl.these six measurements are assumed to be an srs of all possible measurements from solution.they are also assumed to have a standard deviation of 0.2, a normal distributin, and a mean concentration equal to the true concentration of the solution.carry out a significance test to determine whether these six measurements provide reliable evidence that the true concentration of the solution is actually not 5 mg/dl.
Answers: 1

Chemistry, 22.06.2019 23:30
The comparison of the number of atoms in a copper coin the size of a penny with the number of people on earth is made to illustrate which of the following? a. that atoms are indivisible b. that atoms are very small c. that atoms are very large d. that in a copper penny, there is one atom for every person on earth
Answers: 1
You know the right answer?
A5.5 g sample of a substance contains only carbon and oxygen. carbon makes up 35% of the mass of the...
Questions

Mathematics, 26.03.2021 06:50

Mathematics, 26.03.2021 06:50


English, 26.03.2021 06:50

Mathematics, 26.03.2021 06:50

Mathematics, 26.03.2021 06:50








Mathematics, 26.03.2021 06:50

Mathematics, 26.03.2021 06:50

History, 26.03.2021 06:50

History, 26.03.2021 06:50

Mathematics, 26.03.2021 06:50

Mathematics, 26.03.2021 06:50

Mathematics, 26.03.2021 06:50