
Chemistry, 08.10.2019 23:10 peterblessd6868
Which structure correctly represents the bonding in a hydrocarbon molecule?
a.
6 carbon atoms forming single bonds with 13 hydrogen atoms; the second carbon from the right has a methyl group attached to it
b.
8 carbon atoms forming single bonds with 18 hydrogen atoms; a triple bond exists between the third and fourth carbon atom from the right
c.
four carbon atoms forming single bonds with seven hydrogen atoms; a methyl group forms a single bond with the second carbon from the left
d.
three carbon atoms forming single bonds with seven hydrogen atoms with; a double bond exists between the first and second carbon atom from the right

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 09:20
What happened to the amount of carbon dioxide in the atmosphere from 2010–2017?
Answers: 1

Chemistry, 23.06.2019 01:00
Chromium(iii) sulfate is a transition metal compound containing the metal chromium and the polyatomic ion sulfate. the oxidation state of chromium in this compound is , and the chemical formula of the compound is ( ) . reset next
Answers: 3

Chemistry, 23.06.2019 03:00
Achemical equilibrium between gaseous reactants and products is shown. n2(g) + 3h2(g) ⇌ 2nh3(g) how will the reaction be affected if the pressure on the system is increased? it will shift toward the reactant side as there is lower pressure on the reactant side. it will shift toward the product side as there is higher pressure on the product side. it will shift toward the reactant side as there are a greater number of moles of gas on the reactant side. it will shift toward the product side as there are a fewer number of moles of gas on the product side.
Answers: 2

Chemistry, 23.06.2019 03:50
How many liters of oxygen gas, at standardtemperature and pressure, will react with 35.8 grams ofiron metal? 4 fe (s) + 3 o2 (g) → 2 fe2o3 (s)
Answers: 3
You know the right answer?
Which structure correctly represents the bonding in a hydrocarbon molecule?
a.
6...
a.
6...
Questions




Biology, 20.09.2020 04:01


Mathematics, 20.09.2020 04:01




Mathematics, 20.09.2020 04:01

Mathematics, 20.09.2020 04:01

Mathematics, 20.09.2020 04:01

Biology, 20.09.2020 04:01

History, 20.09.2020 04:01

Chemistry, 20.09.2020 04:01

History, 20.09.2020 04:01

Mathematics, 20.09.2020 04:01

Chemistry, 20.09.2020 04:01

Geography, 20.09.2020 04:01

Mathematics, 20.09.2020 04:01

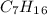 .
.
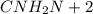 ,Which is the general formula for alkanes.
,Which is the general formula for alkanes.


