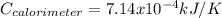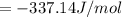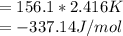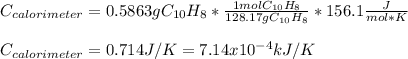When a 1.0560 g benzoic acid sample was burned in a bomb calorimeter to establish the calorimeter constant, a temperature rise of 2.862 k was measured near 298k. under similar conditions, a temperature rise of 2.416 k was measured when a 0.5863 g naphthalene sample was burned. determine the calorimeter constant (in units of kj/k) and the standard enthalpy of combustion for naphthalene at 298k. if we make the assumption that ∆h ≈∆u, is the ∆chº value obtained from this experim

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 10:30
Skills of homo sapiens were found an excavation. the skulls were preserved because the bodies were frozen. so, these fossils are (blank) fossils.the image shows the evolution of skulls beginning 2 to 3 million years ago. based on the image, modern human skulls(blank) ape skulls.
Answers: 1


Chemistry, 23.06.2019 04:00
What is the volume of 2.5 moles of nitrogen gas (n2)at standard temperature and pressure (stp)?
Answers: 1

Chemistry, 23.06.2019 13:30
Elaborate on the reason(s) that matter is said to move even as in a solid state. select one: a. the particles are bound through intermolecular forces but are able to move past each other with relative freedom. b. the particles have sufficient energy to become an ionized gas and are in the most common state of matter in the universe. c. the particles are not able to move out of their positions relative to one another, but do have small vibrational movements. d. the particles are not bound to one another, move quickly, have a low density, and are able to spread apart from one another if unconstrained.
Answers: 1
You know the right answer?
When a 1.0560 g benzoic acid sample was burned in a bomb calorimeter to establish the calorimeter co...
Questions



Mathematics, 20.01.2021 17:40


History, 20.01.2021 17:40



Mathematics, 20.01.2021 17:40

English, 20.01.2021 17:40

Chemistry, 20.01.2021 17:40

Mathematics, 20.01.2021 17:40

Social Studies, 20.01.2021 17:40

Mathematics, 20.01.2021 17:40


Mathematics, 20.01.2021 17:40





History, 20.01.2021 17:40