
Chemistry, 08.10.2019 04:30 bonnysvalentine
Naturally occurring magnesium has an atomic mass of 24.312 and consists of three isotopes. the major isotope is 24mg, natural abundance 78.99%, relative atomic mass 23.98504. the next most abundant isotope is 26mg, relative atomic mass 25.98259. the third most abundant isotope is 25mg whose natural abun- dance is in the ratio of 0.9083 to that of 26mg. find the relative atomic mass of 25mg.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 09:00
This chart lists four kinds of polymers and their sources. what can be known about all four polymers, despite their differences? they come from living things. they share ionic carbon bonds. they are at least 100 monomers long. they are made of repeating subunits.
Answers: 3

Chemistry, 22.06.2019 21:00
Which answer tells the reason the earth’s climate is getting warmer? too many animals are becoming extinct. large glaciers are melting in antarctica. the earth is moving closer to the sun. driving cars gives off gases that trap heat in the atmosphere.
Answers: 1

Chemistry, 23.06.2019 02:00
Which statement is true about the model of the electromagnetic spectrum a: it change the frequencies of light. b: it compare wavelengths of light. c: the color of light waves can be changed using the model. d: the intensities of light waves can be decreased using the model.
Answers: 2

You know the right answer?
Naturally occurring magnesium has an atomic mass of 24.312 and consists of three isotopes. the major...
Questions

Mathematics, 10.12.2020 19:20





English, 10.12.2020 19:20



English, 10.12.2020 19:20

Biology, 10.12.2020 19:20

History, 10.12.2020 19:20


Mathematics, 10.12.2020 19:20

Engineering, 10.12.2020 19:20



Mathematics, 10.12.2020 19:20



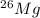 , then 0.9083x represents % for
, then 0.9083x represents % for 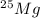 .
.

