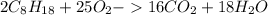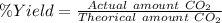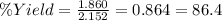Chemistry, 08.10.2019 05:00 justin5647
Using the following equation, determine the % yield from the following reaction if 30.65 g of octane (c_8 8 h_{18} 18 ) react with excess oxygen to produce 81.75 g of co_2 2 (g) 2c_8 8 h_{18} 18 (i) + 25o_2 2 (g) → 16co_2 2 (g) + 18h_2 2 o(l)

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 15:00
20 pts ‼️ an unmanned spacecraft travels to mars. mars has a lower strength of gravity than earth. where in the image is the spacecraft’s weight the greatest?
Answers: 1

Chemistry, 22.06.2019 23:00
What is the oxidation state of each individual carbon atom in c2o42−?
Answers: 1

Chemistry, 23.06.2019 01:00
Which substance—wood or silver—is the better thermal conductor? a thermal conductor is a material that requires very little heat energy to change its temperature. explain your answer.
Answers: 3

Chemistry, 23.06.2019 09:30
Need , hurry pls create a superhero out of the element iron, what are its powers and his sidekick ( an element that works well with iron). how was the superhero made and who discovered him
Answers: 3
You know the right answer?
Using the following equation, determine the % yield from the following reaction if 30.65 g of octane...
Questions

Computers and Technology, 20.07.2021 19:40


Computers and Technology, 20.07.2021 19:40

Computers and Technology, 20.07.2021 19:40



Mathematics, 20.07.2021 19:40

Business, 20.07.2021 19:40

French, 20.07.2021 19:40

Social Studies, 20.07.2021 19:40





Mathematics, 20.07.2021 19:40



Physics, 20.07.2021 19:40


Mathematics, 20.07.2021 19:40