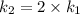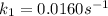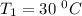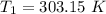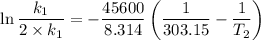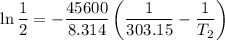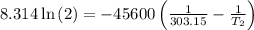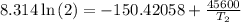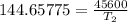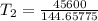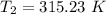Chemistry, 08.10.2019 03:20 jonlandis6
The arrhenius equation shows the relationship between the rate constant k and the temperature t in kelvins and is typically written as k=ae−ea/rt where r is the gas constant (8.314 j/mol⋅k), a is a constant called the frequency factor, and ea is the activation energy for the reaction. however, a more practical form of this equation is lnk2k1=ear(1t1−1t2) which is mathematically equivalent to lnk1k2=ear(1t2−1t1) where k1 and k2 are the rate constants for a single reaction at two different absolute temperatures (t1 and t2). part a the activation energy of a certain reaction is 45.6 kj/mol . at 30 ∘c , the rate constant is 0.0160s−1 . at what temperature in degrees celsius would this reaction go twice as fast

Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 03:30
Asample of ammonia reacts with oxygen as shown. 4nh3(g) + 5o2(g) 4no(g) + 6h2o(g) what is the limiting reactant if 4.0 g of nh3 react with 8.0 g of oxygen? o2 because it produces only 0.20 mol of no. nh3 because it produces only 0.20 mol of no. o2 because it produces two times less no than nh3. nh3 because it produces three times more no than o2.
Answers: 3

Chemistry, 22.06.2019 07:50
Which of the following electromagnetic waves can create ions?
Answers: 2

Chemistry, 22.06.2019 13:00
Jose and eric were given four samples in lab. the results of their analysis are shown in the table. based on the data they collected, which sample is most likely a metal?
Answers: 1
You know the right answer?
The arrhenius equation shows the relationship between the rate constant k and the temperature t in k...
Questions

Mathematics, 13.02.2022 01:00

Mathematics, 13.02.2022 01:00

Biology, 13.02.2022 01:00



Mathematics, 13.02.2022 01:00

Social Studies, 13.02.2022 01:00

Mathematics, 13.02.2022 01:00




Mathematics, 13.02.2022 01:00





Chemistry, 13.02.2022 01:00



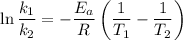
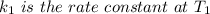
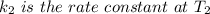
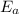 is the activation energy
is the activation energy