
Chemistry, 08.10.2019 02:30 iwantcandy2002
Be sure to answer all parts. sulfur dioxide is released in the combustion of coal. scrubbers use lime slurries of calcium hydroxide to remove the so2 from the flue gases. write the balanced equation for the reaction between solid calcium hydroxide and so2. include the states of all reactants and products in your equation. now, calculate the δs o at 298 k [s o of caso3(s) = 101.4 j/mol k].

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 18:50
Suppose you got a low yield of benzoin from your benzoin condensation reaction and thus only have 0.300 g of benzoin to use as the starting material for this reaction. how much concentrated nitric acid should you add? (concentrated nitric acid is 15.8 m). write your answer in the form x.xx ml
Answers: 1

Chemistry, 22.06.2019 04:00
You encounter a solution that is acidic and you decide to test it by adding a small amount of a strong acid. the ph lowers slightly but is approximately unchanged, and still remains acidic. what can you say about the solution? a. it is a buffer solution. b. it is not a buffer solution it is a strong acid solution. d. the solution has been neutralized. e. the solution has excess acid present
Answers: 1

Chemistry, 22.06.2019 04:00
Tin has ten stable isotopes. the heaviest, 124sn, makes up 5.80% of naturally occuring tin atoms. how many atoms of 124sn are present in 82.0 g of naturally occurring tin? what is the total mass of the 124sn atoms in this sample?
Answers: 3

Chemistry, 22.06.2019 06:00
The tilt of the earth's axis of rotation is responsible for the a) ocean's tides. b) size of the moon. c) brightness of stars. d) earth’s seasons.
Answers: 1
You know the right answer?
Be sure to answer all parts. sulfur dioxide is released in the combustion of coal. scrubbers use lim...
Questions







Physics, 09.03.2020 20:01





Geography, 09.03.2020 20:02



History, 09.03.2020 20:03





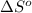 for the reaction is -160.6 J/K
for the reaction is -160.6 J/K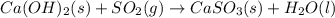
![\Delta S^o_{rxn}=\sum [n\times \Delta S^o_{products}]-\sum [n\times \Delta S^o_{reactants}]](/tpl/images/0298/9458/e71e2.png)
![\Delta S^o_{rxn}=[(1\times \Delta S^o_{CaSO_3(s)})+(1\times \Delta S^o_{H_2O(l)})]-[(1\times \Delta S^o_{Ca(OH)_2(s)})+(1\times \Delta S^o_{SO_2(g)})]](/tpl/images/0298/9458/6c149.png)
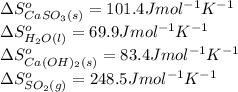
![\Delta S^o_{rxn}=[(1\times (101.4))+(1\times (69.9))]-[(1\times (83.4))+(1\times (248.5))]\\\\\Delta S^o_{rxn}=-160.6J/K](/tpl/images/0298/9458/59a05.png)


