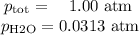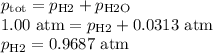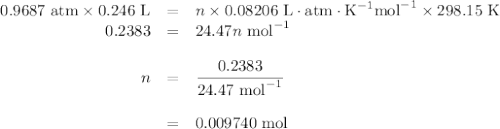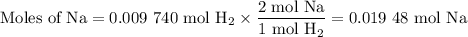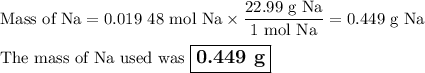Chemistry, 08.10.2019 02:10 lazymarshmallow7
Apiece of sodium metal reacts completely with water as follows: 2na(s) + 2h2o(l) ⟶ 2naoh(aq) + h2(g) the hydrogen gas generated is collected over water at 25.0°c. the volume of the gas is 246 ml measured at 1.00 atm. calculate the number of grams of sodium used in the reaction. (vapor pressure of water at 25°c = 0.0313 atm.)

Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 07:30
Aradio signal from a gps satellite take only about 0.067 seconds to reach a gps reciever. if the speed of light is about 300,000km/s, then approximately how far away is the reciever from from the satellite?
Answers: 1

Chemistry, 22.06.2019 08:00
Will give ! what are the advantages and disadvantages of nuclear power? check all that apply. one advantage of nuclear energy is that it does not produce carbon dioxide emissions. storage of nuclear waste is a short-term problem associated with nuclear energy. the problem with uranium mining is that a large quantity of uranium must be extracted to meet energy needs because the energy release from uranium fission is so low. safe operation of a nuclear power plant can be jeopardized by a human mistake.
Answers: 1

Chemistry, 22.06.2019 14:00
Calculate the frequency of a wave in a spring toy. the wave has a speed of 1.1 meters per second and a wavelength of 0.1 meters. *
Answers: 2
You know the right answer?
Apiece of sodium metal reacts completely with water as follows: 2na(s) + 2h2o(l) ⟶ 2naoh(aq) + h2(g...
Questions





Mathematics, 30.10.2021 01:10



Geography, 30.10.2021 01:10

Biology, 30.10.2021 01:10





SAT, 30.10.2021 01:10

English, 30.10.2021 01:10



Mathematics, 30.10.2021 01:10

Mathematics, 30.10.2021 01:10