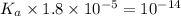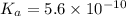Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 20:00
Which object forms when a supergiant runs out of fuel? a red giant a black hole a white dwarf a neutron star
Answers: 1

Chemistry, 21.06.2019 21:30
Aphysical reaction is a process in which one or more reactants change into one or more products with different properties. select the best answer from the choices provided t f
Answers: 1

Chemistry, 22.06.2019 10:40
Which buffer would be better able to hold a steady ph on the addition of strong acid, buffer 1 or buffer 2? explain. buffer 1: a solution containing 0.10 m nh4cl and 1 m nh3. buffer 2: a solution containing 1 m nh4cl and 0.10 m nh3
Answers: 1

Chemistry, 22.06.2019 12:20
Consider the reaction of a(g) + b(g) + c(g) => d(g) for which the following data were obtained: experiment initial [a], mol/l initial [b], mol/l initial [c], mol/l initial rate, mol/l.s 1 0.0500 0.0500 0.0100 6.25 x 10^-3 2 0.100 0.0500 0.0100 2.50 x 10^-2 3 0.100 0.100 0.0100 1.00 x 10^-1 4 0.0500 0.0500 0.0200 6.25 x 10^-3 what is the rate law for the reaction?
Answers: 3
You know the right answer?
Given that at 25.0 ∘c ka for hcn is 4.9×10−10 and kb for nh3 is 1.8×10−5, calculate kb for cn− and k...
Questions

Mathematics, 09.12.2020 20:40


Mathematics, 09.12.2020 20:40

Mathematics, 09.12.2020 20:40

History, 09.12.2020 20:40



History, 09.12.2020 20:40




Mathematics, 09.12.2020 20:40

Mathematics, 09.12.2020 20:40

Mathematics, 09.12.2020 20:40

History, 09.12.2020 20:40





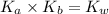
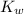 is the dissociation constant of water.
is the dissociation constant of water.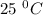 ,
, 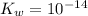
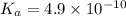
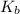 for CN⁻ can be calculated as:
for CN⁻ can be calculated as:
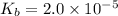
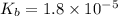
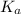 for
for 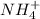 can be calculated as:
can be calculated as: