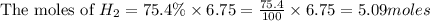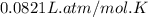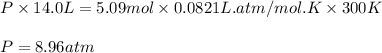Chemistry, 07.10.2019 23:00 brandytyler317fries
Consider 2 al + 6 hcl → 2 alcl3 + 3 h2 , the reaction of al with hcl to produce hydrogen gas. what is the pressure of h2 if the hydrogen gas collected occupies 14.0 l at 300.k and was produced upon reaction of 4.50 moles of al and excess hcl in a process that has a 75.4 percent yield?

Answers: 2


Another question on Chemistry




Chemistry, 22.06.2019 22:30
Molecular iodine, i2(g), dissociates into iodine atoms at 625 k with a first-order rate constant of 0.271 s−1. part a part complete what is the half-life for this reaction?
Answers: 3
You know the right answer?
Consider 2 al + 6 hcl → 2 alcl3 + 3 h2 , the reaction of al with hcl to produce hydrogen gas. what i...
Questions

Biology, 23.07.2019 19:30




Social Studies, 23.07.2019 19:30

Social Studies, 23.07.2019 19:30

Mathematics, 23.07.2019 19:30

English, 23.07.2019 19:30

Mathematics, 23.07.2019 19:30




Mathematics, 23.07.2019 19:30

Biology, 23.07.2019 19:30

Spanish, 23.07.2019 19:30

History, 23.07.2019 19:30

Social Studies, 23.07.2019 19:30


Mathematics, 23.07.2019 19:30

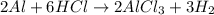
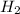 gas
gas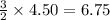 moles of
moles of