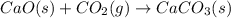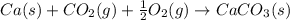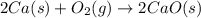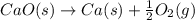Consider the equations below. (1) ca(s) + co2(g) + 1 2 o2(g) → caco3(s) (2) 2ca(s)+o2(g) → 2cao(s) how should you manipulate these equations so that they produce the equation below when added? check all that apply. cao(s) + co2(g) → caco3(s) reverse the direction of equation (2) multiply equation (1) by 3 multiply equation (2) by 1/2

Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 15:30
The reactions of photosynthesis occur in the of plant cell? a.mitochondria. b. lysosomes. c. chloroplasts. d. chlorophyll
Answers: 1

Chemistry, 22.06.2019 22:30
The vapor pressure of ethanol is 1.00 × 102 mmhg at 34.90°c. what is its vapor pressure at 61.61°c? (δhvap for ethanol is 39.3 kj/mol.)
Answers: 2

Chemistry, 22.06.2019 23:30
The density of the solid phase of a substance is 0.90 g/cm3 and the density of the liquid phase is 1.0 g/cm3. a large increase in pressure will a. lower the freezing point b. raise the freezing point c. lower the boiling point d. raise the triple point e. lower the triple point
Answers: 1
You know the right answer?
Consider the equations below. (1) ca(s) + co2(g) + 1 2 o2(g) → caco3(s) (2) 2ca(s)+o2(g) → 2cao(s) h...
Questions



Social Studies, 17.11.2021 09:00

Mathematics, 17.11.2021 09:00




Business, 17.11.2021 09:00




History, 17.11.2021 09:10


Mathematics, 17.11.2021 09:10

Mathematics, 17.11.2021 09:10


English, 17.11.2021 09:10

History, 17.11.2021 09:10

History, 17.11.2021 09:10