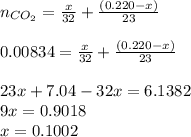An organic liquid is a mixture of methyl alcohol (ch3oh m ch_3oh) and ethyl alcohol (c2h5oh m c_2h_5oh ). a 0.220-g m g sample of the liquid is burned in an excess of o2(g) m o_2(g) and yields 0.367g g co2(g) m co_2(g) (carbon dioxide). set up two algebraic equations, one expressing the mass of carbon dioxide produced in terms of each reagent and the other expressing the mass of sample burned in terms of each reagent.
what is the mass of methyl alcohol (ch3oh m ch_3oh) in the sample?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 06:00
According to each substances heat of fusion, which of the items below requires more heat to be added per gram of substance to go from solid to liquid? silver sulfur water lead
Answers: 2

Chemistry, 22.06.2019 15:20
An alloy contains 66 g of pure zinc. what is the percentage of zinc in the alloy? express your answer to two significant figures and include the appropriate units.
Answers: 1

Chemistry, 22.06.2019 22:30
Which process describes vaporization that takes place below the surface of a liquid? condensation melting boiling evaporation
Answers: 1

Chemistry, 22.06.2019 22:40
Percent ionization for a weak acid (ha) is determined by the following formula: percent ionization=[ha] ionized[ha] initial×100%for strong acids, ionization is nearly complete (100%) at most concentrations. however, for weak acids, the percent ionization changes significantly with concentration. the more diluted the acid is, the greater percent ionization.a certain weak acid, ha, has a ka value of 9.4×10? 7.part acalculate the percent ionization of ha in a 0.10 m solution.part bcalculate the percent ionization of ha in a 0.010 m solution
Answers: 1
You know the right answer?
An organic liquid is a mixture of methyl alcohol (ch3oh m ch_3oh) and ethyl alcohol (c2h5oh m c_2h_5...
Questions









English, 07.10.2021 02:20


Mathematics, 07.10.2021 02:20

Computers and Technology, 07.10.2021 02:20

Biology, 07.10.2021 02:20

Computers and Technology, 07.10.2021 02:20

English, 07.10.2021 02:20





Computers and Technology, 07.10.2021 02:20

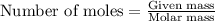 .....(1)
.....(1)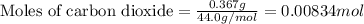
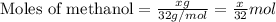
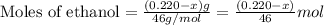
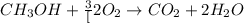
![\frac{x}{32} moles of methanol will produce = [tex]\frac{1}{1}\times \frac{x}{32}=\frac{x}{32}](/tpl/images/0297/8500/66d68.png) moles of carbon dioxide
moles of carbon dioxide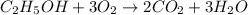
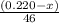 moles of ethanol will produce =
moles of ethanol will produce = 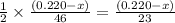 moles of carbon dioxide
moles of carbon dioxide