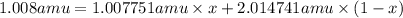There are two isotopes of hydrogen commonly found in nature. hydrogen-1 which has an atomic mass of 1.007751 amu and hydrogen-2 which has an atomic mass of 2.014741. which isotope of these two isotopes is the most abundant? hint: think about how the atomic mass of the element hydrogen is calculated.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 21:00
Which statement describes evidence of a chemical reaction? a) ice melting eliminate b) water boiling c) lighting a match d) grape juice freezing
Answers: 3

Chemistry, 21.06.2019 21:10
Harvey mixes two liquids. which observation of the new mixture most likely indicates a precipitate is forming?
Answers: 2

Chemistry, 22.06.2019 06:30
Select the correct text in the passage. which sentences describe examples of sustainable living? i live in an old apartment building downtown, but my company is based in an office park on the outskirts of the city. i drive an old car that needs to be replaced. i plan to buy a hybrid for better gas mileage, but for now i am able to carpool with a couple of friends from work. the drive to the office park is about 45 minutes each way, but we do get to work in a modern building. the architects just received a leed certification for the design.
Answers: 3

Chemistry, 22.06.2019 20:30
Calculate the percent composition by mass of each element in al(oh)3. use at least three significant figures.
Answers: 1
You know the right answer?
There are two isotopes of hydrogen commonly found in nature. hydrogen-1 which has an atomic mass of...
Questions




Mathematics, 30.06.2019 21:00


Mathematics, 30.06.2019 21:00


Mathematics, 30.06.2019 21:00


Health, 30.06.2019 21:00

Mathematics, 30.06.2019 21:00

Mathematics, 30.06.2019 21:00

Social Studies, 30.06.2019 21:00


Mathematics, 30.06.2019 21:00

Mathematics, 30.06.2019 21:00

Mathematics, 30.06.2019 21:00



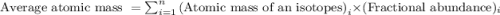 ....(1)
....(1)