
Chemistry, 07.10.2019 18:30 oliviaclerk5
Hector claims that a single sodium ion and a single oxygen ion do not bond together to form a stable binary ionic compound. is he correct? why or why not?
a hector is incorrect. the two ions have opposite charges, so they will bond together in a binary ionic compound.
b hector is incorrect. the two ions have more than eighteen protons, so they will bond together in a binary ionic compound.
c hector is correct. the sum of the charges of the two ions must be zero in order for them to form a stable ionic compound.
d hector is correct. the sum of the protons of the two ions must be divisible by eight in order for them to form a stable ionic compound.

Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 02:50
The conventional equilibrium constant expression (kc) for the system below is: 2icl(s) ⇄ i2(s) + cl2(g) [cl2] ([i2] + [cl2])/2[icl] [i2][cl2]/[icl]2 none of the listed answers are correct [i2][cl2]/2[icl]
Answers: 2

Chemistry, 22.06.2019 05:30
Why is soap used to remove grease? a. its nonpolar end dissolves the grease. b. it makes the water bond with the grease. c. it chemically bonds with the grease. d. its polar end dissolves the grease.correct answer for apex - a, its nonpolar end dissolves the grease.
Answers: 1

Chemistry, 22.06.2019 07:30
Compare and contrast the bohr model and the electron cloud models of the atom.
Answers: 1
You know the right answer?
Hector claims that a single sodium ion and a single oxygen ion do not bond together to form a stable...
Questions

Mathematics, 16.12.2020 18:10


Biology, 16.12.2020 18:10

Mathematics, 16.12.2020 18:10

Chemistry, 16.12.2020 18:10

Mathematics, 16.12.2020 18:10




Mathematics, 16.12.2020 18:10


English, 16.12.2020 18:10



Mathematics, 16.12.2020 18:10

Chemistry, 16.12.2020 18:10




![[Na]=1s^22s^22p^63s^1](/tpl/images/0297/6892/4ee3b.png)
![[O]=1s^22s^22p^4](/tpl/images/0297/6892/336ff.png)
![[O^{2-}]=1s^22s^22p^6](/tpl/images/0297/6892/c797e.png)
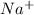 cation and oxide
cation and oxide 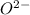 is an anion with oxidation state of -2. Thus they combine and their oxidation states are exchanged and written in simplest whole number ratios to give neutral
is an anion with oxidation state of -2. Thus they combine and their oxidation states are exchanged and written in simplest whole number ratios to give neutral 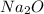 .
.

