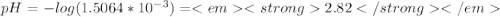Chemistry, 07.10.2019 17:30 justachelseafan
Weak acids and bases are those that do not completely dissociate in water. the dissociation of the acid or base is an equilibrium process and has a corresponding equilibrium constant. ka is the equilibrium constant for the dissociation of a weak acid and kb is the equilibrium constant for the dissociation of a weak base. what is the ph of a solution that has 0.125 m ch3cooh and 0.125 m h3bo3? ka of ch3cooh = 1.8 × 10−5 and ka of h3bo3 = 5.4 × 10−10 answer unselected 4.74 unselected 5.64 unselected 2.82 unselected 2.52 unselected

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 08:20
What is the formula for the compound dinitrogen pentoxide? a. n4o5 b. n5o4 c. n4o6 d. n5o2 e. n2o5
Answers: 3

Chemistry, 22.06.2019 11:30
Aperfume bottle is dropped in the corner of a room. the odor of the perfume can be detected on the other side of the room. which statement best describes this observation?
Answers: 2

Chemistry, 22.06.2019 12:30
Which of the following describes a compound? (hint: carbon and oxygen bo a. a piece of pure carbon, containing only carbon atoms b. oxygen gas surrounding a solid piece of carbon c. a substance made of two oxygen atoms for each carbon atom carbon and oxygen atoms mixed without being bonded together
Answers: 1

Chemistry, 22.06.2019 17:00
The msds for glacial acetic acid says that it is a flammable liquid that can severely burn any human tissue it comes in contact with. it reacts with bases, various metals, and strong oxidizing agents. its vapors can form explosive mixtures with air.
Answers: 1
You know the right answer?
Weak acids and bases are those that do not completely dissociate in water. the dissociation of the a...
Questions



Mathematics, 26.06.2020 21:01




Mathematics, 26.06.2020 21:01


Social Studies, 26.06.2020 21:01

Mathematics, 26.06.2020 21:01





Mathematics, 26.06.2020 21:01


English, 26.06.2020 21:01


Mathematics, 26.06.2020 21:01

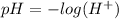 Equation (1)
Equation (1)
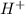 , because the proton is transferred to
, because the proton is transferred to 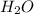 to form hydronium ions,
to form hydronium ions, 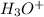 . Thus, we can determine the pH of the solution, finding the molar concentration of
. Thus, we can determine the pH of the solution, finding the molar concentration of 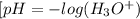 Equation (2)
Equation (2)
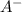
![k= \frac{[H_3O^{+}][A^{-}]}{[HA][H_2O]}](/tpl/images/0297/5192/1a08e.png) Equation (3)
Equation (3)
![k_a=K[H_2O]= \frac{[H_3O^{+}][A^{-}]}{[HA]}](/tpl/images/0297/5192/38c8c.png) Equation (4)
Equation (4)
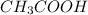 :
:
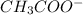
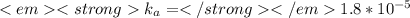
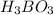 :
:
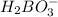
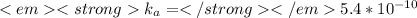
![1.8*10^{-5} =\frac{[x][x]}{[0.125-x]}](/tpl/images/0297/5192/6d8ce.png) Equation (5)
Equation (5)
![5.4*10^{-10} =\frac{[y][y]}{[0.125-y]}](/tpl/images/0297/5192/98489.png) Equation (6)
Equation (6)![x = [H_{3}O^{+}] = 1.49*10^{-3}](/tpl/images/0297/5192/56324.png)
![y = [H_{3}O^{+}] = 1.64*10^{-6}](/tpl/images/0297/5192/edda0.png)
![[H_{3}O^{+}]](/tpl/images/0297/5192/71b58.png) is (x+y)
is (x+y) 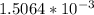 . Replacing this concentration in equation 2, we get:
. Replacing this concentration in equation 2, we get: