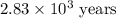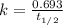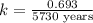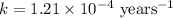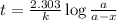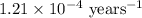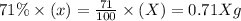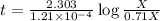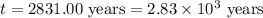Chemistry, 07.10.2019 17:30 animationfusion
Carbon-14 is a radioactive isotope that decays according to first-order kinetics in a process that has a half-life of 5730 years. if a sample containing carbon-14 now has 71% of its original concentration of carbon-14, how much time has passed in years? 4.09 ~ 103 years 5.73 x 103 years 2.38 x 103 years 2.83 x 103 years 3.52 * 104 years

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 12:10
If a molecule with a molecular formula of c13h18 is treated with an excess of h2 in the presence of finally divided pt metal under conditions required for maximum hydrogenation of the molecule to give a molecule with a formula c13h24, how many rings are in the molecule?
Answers: 3

Chemistry, 22.06.2019 14:00
Which of the following is true about a carbonated soft drink? . the carbon dioxide is the solvent, and water is the solute.. the water is the solution, and carbon dioxide is the solvent.. the carbon dioxide is the solution, and the water is the solvent.. the water is the solvent, and the carbon dioxide is the solute.. .
Answers: 1

Chemistry, 23.06.2019 01:30
Select the correct answer from each drop-down menu. to make a table of the elements, dmitri mendeleev sorted the elements according to their . he then split the list of elements into several columns so that elements beside each other had similar .
Answers: 2

Chemistry, 23.06.2019 01:30
What is produced from neutralization of an acid and a base? a. hydronium ions b. citric acid c. salt and water
Answers: 1
You know the right answer?
Carbon-14 is a radioactive isotope that decays according to first-order kinetics in a process that h...
Questions


Computers and Technology, 26.08.2020 01:01


Social Studies, 26.08.2020 01:01

Mathematics, 26.08.2020 01:01






Mathematics, 26.08.2020 01:01





Mathematics, 26.08.2020 01:01