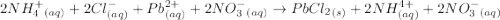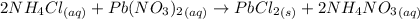Chemistry, 07.10.2019 16:20 shaylawaldo11
When a solution of ammonium chloride, nh4cl, is added to a solution of lead (ii) nitrate , pb(no3)2, a white precipitate, pbcl2, forms. which of the following is the total ionic equation for this reaction?
a) 2 nh4cl(aq)+pb(no3)2(> pbcl2(s)+2 nh4no3 (aq)
b) 2 nh4+(aq)+2cl-(aq)+pb2+(aq)+2no3-(&g t; pbcl2(s)+2nh4+(aq)+2no3-(aq)
c) pb2+(aq)+2cl-(> pbcl2(s)
d) pb2+(aq)+cl2-(> pbcl2(s)

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 20:50
Choose all that apply. when creating a graph, you should: determine the x- and y- variables label the scale on the x- and y- axes plot the data points draw a line of best fit to represent the data trend
Answers: 1

Chemistry, 22.06.2019 03:30
Calculate the molar mass of aluminum oxide (al2o3). express your answer to four significant figures.
Answers: 1

Chemistry, 23.06.2019 01:00
Na chemical reaction, activation energy increases the of the reactants. this outcome causes the particles to collide, which results in the of new products.
Answers: 2

Chemistry, 23.06.2019 03:00
Achemical equilibrium between gaseous reactants and products is shown. n2(g) + 3h2(g) ⇌ 2nh3(g) how will the reaction be affected if the pressure on the system is increased? it will shift toward the reactant side as there is lower pressure on the reactant side. it will shift toward the product side as there is higher pressure on the product side. it will shift toward the reactant side as there are a greater number of moles of gas on the reactant side. it will shift toward the product side as there are a fewer number of moles of gas on the product side.
Answers: 2
You know the right answer?
When a solution of ammonium chloride, nh4cl, is added to a solution of lead (ii) nitrate , pb(no3)2,...
Questions

Mathematics, 19.02.2021 14:00

Mathematics, 19.02.2021 14:00


English, 19.02.2021 14:00


English, 19.02.2021 14:00

History, 19.02.2021 14:00

Mathematics, 19.02.2021 14:00

Mathematics, 19.02.2021 14:00


Mathematics, 19.02.2021 14:00

History, 19.02.2021 14:00

Mathematics, 19.02.2021 14:00


Arts, 19.02.2021 14:00


Mathematics, 19.02.2021 14:00