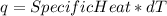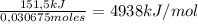Chemistry, 07.10.2019 16:10 dillondelellis2006
Consider the reaction c12h22o11 (s) + 12 o2 (g) → 12 co2 (g) + 11 h2o (l) in which 10.5 g of sucrose, c12h22o11, was burned in a bomb calorimeter with a heat capacity of 7.50 kj/oc (including its water). the temperature inside the calorimeter was found to increase by 20.2 oc. based on this information, what is the heat of this reaction per mole of sucrose?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 04:00
Two nitro no2 groups are chemically bonded to a patch of surface. they can't move to another location on the surface, but they can rotate (see sketch at right). it turns out that the amount of rotational kinetic energy each no2 group can have is required to be a multiple of ε, where =ε×1.010−24 j. in other words, each no2 group could have ε of rotational kinetic energy, or 2ε, or 3ε, and so forth — but it cannot have just any old amount of rotational kinetic energy. suppose the total rotational kinetic energy in this system is initially known to be 32ε. then, some heat is removed from the system, and the total rotational kinetic energy falls to 18ε. calculate the change in entropy. round your answer to 3 significant digits, and be sure it has the correct unit symbol.
Answers: 2

Chemistry, 22.06.2019 10:30
Use this information to determine the number of calends electrons in the atoms. which of the following correctly compares the stability of the two atoms? a) both are unreactive b) both are highly reactive c) a is unreactive and d is reactive d) a is reactive and d is unreactive
Answers: 2

Chemistry, 22.06.2019 13:00
One of the hopes for solving the world's energy problem is to make use of the fusion reaction 21h +31h --> 42he + 10n + energy how much energy is released when 1 mol of deuterium is fused with 1 mol of tritium according to the above reaction? the masses of the atoms and the neutrons are as follows: 21h = 2.0140 amu 31h = 3.01605 amu 42he = 4.002603 amu 10n = 1.008665 amu. the speed of light is 2.9979 x 108 m/s.
Answers: 1

Chemistry, 23.06.2019 06:00
What volume of 0.500 mol/l hydrochloric acid, hci (aq) is required to react completely with 1.00 g of aluminum hydroxide, ai(oh)3 (s)?
Answers: 1
You know the right answer?
Consider the reaction c12h22o11 (s) + 12 o2 (g) → 12 co2 (g) + 11 h2o (l) in which 10.5 g of sucrose...
Questions

Mathematics, 10.03.2021 23:40

Mathematics, 10.03.2021 23:40


Mathematics, 10.03.2021 23:40


Mathematics, 10.03.2021 23:40

English, 10.03.2021 23:40

Mathematics, 10.03.2021 23:40

Arts, 10.03.2021 23:40

Mathematics, 10.03.2021 23:40



Mathematics, 10.03.2021 23:40

Mathematics, 10.03.2021 23:40






English, 10.03.2021 23:40