
Chemistry, 06.10.2019 19:30 opgbadwolf5
Ascorbic acid, or vitamin c (c6h8o6, molar mass = 176 g/mol), is a naturally occurring organic compound with antioxidant properties. a healthy adult’s daily requirement of vitamin c is 70-90 mg. a sweet lime contains 2.82×10−4 mol of ascorbic acid.
to determine whether the ascorbic acid in a sweet lime meets the daily requirement, calculate the mass of ascorbic acid in 2.82×10−4 mol of ascorbic acid.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 06:30
Particle model to predict what will happen if a sharp object creates a hole in the soccer ball
Answers: 2

Chemistry, 22.06.2019 15:00
‘which reaction would most likely require the use of an inert electrode?
Answers: 1

Chemistry, 22.06.2019 20:30
Which of the following is not true about the atomic model of substances?
Answers: 1

Chemistry, 22.06.2019 21:30
What is happening when the water inside a kettle heats up and begins to boil
Answers: 1
You know the right answer?
Ascorbic acid, or vitamin c (c6h8o6, molar mass = 176 g/mol), is a naturally occurring organic compo...
Questions

History, 15.11.2020 22:30


Mathematics, 15.11.2020 22:30


History, 15.11.2020 22:30


Social Studies, 15.11.2020 22:30

Social Studies, 15.11.2020 22:30


Mathematics, 15.11.2020 22:30






Physics, 15.11.2020 22:30

Mathematics, 15.11.2020 22:30

Arts, 15.11.2020 22:30


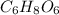 ) contains 6, C atoms 8, H atoms and 6, O atoms .
) contains 6, C atoms 8, H atoms and 6, O atoms .
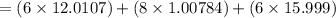
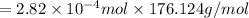
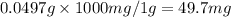 VITAMIN C
VITAMIN C


