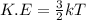Chemistry, 06.10.2019 13:30 tressasill
tori is studying a chemical reaction and its reaction rate after observing the initial rate she adds thermal energy to the reaction. which of the answer choices correctly describes how the addition of thermal energy will affect the reaction rate ?
a.) increase in energy will cause the reactance particles to move faster which will increase their temperature and lead to a faster reaction rate
b.) increase in energy will cause the reactants particles to move faster which will increase their temperature and lead to a slower reaction rate
c.) the increasing energy will cause the reactance particles to move faster which will decrease their temperature and lead to a faster reaction rate
d.) the increasing energy will cause the reactance particles to move slower which will decrease their temperature and lead to a slower reaction rate
e.) increase in energy will cause the reactants particles to move slower which will decrease their temperature and lead to a faster reaction rate

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 21:40
A5 mole sample of liquid acetone is converted to a gas at 75.0°c. if 628 j are required to raise the temperature of the liquid to the boiling point, 15.600 kj are required to evaporate the liquid, and 712 j are required to raise the final temperature to 75.0°c, what is the total energy required for the conversion?
Answers: 3

Chemistry, 23.06.2019 05:30
According to thomson, the atom is a positively charged cloud with electrons scattered throughout. what would the alpha particles do when they hit the foil if thomson were correct
Answers: 1

Chemistry, 23.06.2019 06:00
If you try to move a piano and are unable to move it, did you perform any work in the scientific sense of the word? yes? or no? this question is worth 20 points! let it be correct!
Answers: 1

Chemistry, 23.06.2019 14:50
Use the bond energies provided to estimate δh°rxn for the reaction below. ch3oh(l) + 2 o2(g) → co2(g) + 2 h2o(g) δh°rxn = ? bond bond energy (kj/mol) c-h 414 c-o 360 c=o 799 o=o 498 o-h 464 use the bond energies provided to estimate δh°rxn for the reaction below. ch3oh(l) + 2 o2(g) → co2(g) + 2 h2o(g) δh°rxn = ? bond bond energy (kj/mol) c-h 414 c-o 360 c=o 799 o=o 498 o-h 464 +473 kj +206 kj -392 kj -91 kj -486 kj
Answers: 1
You know the right answer?
tori is studying a chemical reaction and its reaction rate after observing the initial rate she adds...
Questions


Health, 09.12.2020 01:40

History, 09.12.2020 01:40

Mathematics, 09.12.2020 01:40


Mathematics, 09.12.2020 01:40


Mathematics, 09.12.2020 01:40

Arts, 09.12.2020 01:40




Mathematics, 09.12.2020 01:40

Biology, 09.12.2020 01:40


Mathematics, 09.12.2020 01:40

Mathematics, 09.12.2020 01:40


Physics, 09.12.2020 01:40

Mathematics, 09.12.2020 01:40