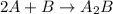Amultistep reaction can only occur as fast as its slowest step. therefore, it is the rate law of the slow step that determines the rate law for the overall reaction. consider the following multistep reaction: a + b → ab (slow) a + ab → a2b (fast)2a + b→ a2b (overall) based on this mechanism, determine the rate law for the overall reaction.

Answers: 2


Another question on Chemistry


Chemistry, 21.06.2019 20:30
If 10.g of agno3 is available, what volume of 0.25 m agno3 can be prepared
Answers: 1

Chemistry, 22.06.2019 07:00
How heavy is thanos? a) 3000 lbs b) all of it c) the price of tea in china d) heavy enough
Answers: 2

Chemistry, 22.06.2019 13:00
If two objects at different te,peraure are in contact with each other what happens to their temperature
Answers: 1
You know the right answer?
Amultistep reaction can only occur as fast as its slowest step. therefore, it is the rate law of the...
Questions






Mathematics, 08.07.2021 20:40

Mathematics, 08.07.2021 20:40

Mathematics, 08.07.2021 20:40



Computers and Technology, 08.07.2021 20:40



History, 08.07.2021 20:40




Health, 08.07.2021 20:40


Mathematics, 08.07.2021 20:40

![Rate=k[A][B]](/tpl/images/0288/1503/27e48.png)
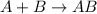 (slow)
(slow)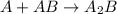 (fast)
(fast)