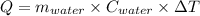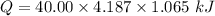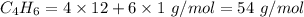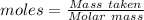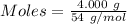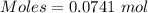Chemistry, 06.10.2019 10:01 Jazzy4real
4.000 g of compound x with molecular formula c4h6 are burned in a constant-pressure calorimeter containing 40.00 kg of water at 25 °c. the temperature of the water is observed to rise by 1.065 °c. (you may assume all the heat released by the reaction is absorbed by the water, and none by the calorimeter itself.) calculate the standard heat of formation of compound x at 25 °c. be sure your answer has a unit symbol, if necessary, and round it to the correct number of significant digits.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 05:30
What royal scientist used the 29th day of frozen vapor to encounter elements for mastering new culinary creations?
Answers: 1

Chemistry, 22.06.2019 06:00
Compare and contrast physical changes with chemical changes.
Answers: 3

Chemistry, 22.06.2019 15:50
How many moles of potassium hydroxide are needed to completely react with 2.94 moles of aluminum sulfate
Answers: 1

Chemistry, 22.06.2019 22:00
11) burning your hand when accidentally touching a hot plate is an example of which heat transfer? a. conduction b. convection c. radiation d. none of these
Answers: 2
You know the right answer?
4.000 g of compound x with molecular formula c4h6 are burned in a constant-pressure calorimeter cont...
Questions

Biology, 06.10.2019 04:01




Mathematics, 06.10.2019 04:01

Chemistry, 06.10.2019 04:01

Mathematics, 06.10.2019 04:01

Biology, 06.10.2019 04:01

Physics, 06.10.2019 04:01

Mathematics, 06.10.2019 04:01

Mathematics, 06.10.2019 04:01

Mathematics, 06.10.2019 04:01

Health, 06.10.2019 04:01


English, 06.10.2019 04:10

Mathematics, 06.10.2019 04:10


History, 06.10.2019 04:10

Mathematics, 06.10.2019 04:10

 = 1.065 °C
= 1.065 °C