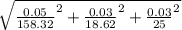Atitration is performed to calculate the concentration of a solution of a monoprotic acid. the buret is filled with a standardized solution of 158.32 ± 0.05 mm naoh. the initial volume is recorded as 0.14 ml. 25.00 ml of the unknown acid solution are pipetted into an erlenmeyer flask and the solution is titrated to a phenolphthalein endpoint. the final buret reading is 18.76 ml. assuming that the error in each volumetric measurement (buret and pipet) is ±0.03 ml, calculate the concentration of the acid (mm) and use propagation of error to estimate its uncertainty.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 03:00
Compare the valence electron configuration of the nobles gas elements seen here. what statement is correct?
Answers: 2

Chemistry, 22.06.2019 09:30
1. explain hydrogen peroxide, h 2 o 2 properties and decomposition reaction. 2. describe how each of the following natural cycles plays a part in earth’s climate system. (a) the water cycle (b) the carbon cycle
Answers: 1

Chemistry, 22.06.2019 12:30
Avariable that is not being directly tested during an experiment should be changed varied experimented controlled
Answers: 1

Chemistry, 22.06.2019 19:20
The equation picture below shows which type of nuclear reaction u 235 + n x e 134 + sr 100 + 2n
Answers: 1
You know the right answer?
Atitration is performed to calculate the concentration of a solution of a monoprotic acid. the buret...
Questions



Social Studies, 23.06.2020 10:57

Chemistry, 23.06.2020 10:57


Mathematics, 23.06.2020 10:57

Mathematics, 23.06.2020 10:57




History, 23.06.2020 10:57






Mathematics, 23.06.2020 10:57



Mathematics, 23.06.2020 10:57