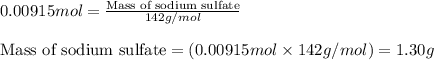Chemistry, 06.10.2019 09:02 eastonstelter
One step in the isolation of pure rhodium metal (rh) is the precipitation of rhodium(iii) hydroxide from a solution containing rhodium(iii) sulfate according to the following balanced chemical equation: rh₂(so₄)₃(aq) + 6naoh(aq) → 2rh(oh)₃(s) + 3na₂so₄(aq) of 0.730 g of rhodium(iii) hydroxide is produced, what mass of sodium sulfate is also produced?

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 17:00
Why do sodium and neon have vastly different chemical and physical properties despite having similar atomic masses?
Answers: 2

Chemistry, 22.06.2019 05:40
Why did southern business leaders want to increase the number of slaves
Answers: 1

Chemistry, 22.06.2019 13:10
Select the correct answer a modure consists of glucose and water. what is the percent composition of glucose in the mixture if it contains 1.3 moles of glucose (cho total mass of the mature is 276 grams? ) and the a 1775
Answers: 1

Chemistry, 22.06.2019 18:30
The table lists the lattice energies of some compounds.compoundlattice energy (kj/mol)lif –1,036licl –853naf –923kf –821nacl –786which statement about crystal lattice energy is best supported by the information in the table? the lattice energy increases as cations get smaller, as shown by lif and kf.the lattice energy increases as the cations get larger, as shown by lif and licl.the lattice energy decreases as cations get smaller, as shown by nacl and naf.the lattice energy decreases as the cations get smaller, as shown by naf and kf.
Answers: 3
You know the right answer?
One step in the isolation of pure rhodium metal (rh) is the precipitation of rhodium(iii) hydroxide...
Questions

History, 17.10.2019 00:30

History, 17.10.2019 00:30

Mathematics, 17.10.2019 00:30


Computers and Technology, 17.10.2019 00:30




Mathematics, 17.10.2019 00:30

Mathematics, 17.10.2019 00:30

Mathematics, 17.10.2019 00:30


History, 17.10.2019 00:30

Engineering, 17.10.2019 00:30

Mathematics, 17.10.2019 00:30


Mathematics, 17.10.2019 00:30



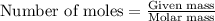 .....(1)
.....(1)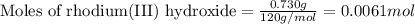
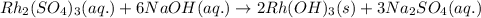
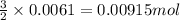 of sodium sulfate is also produced
of sodium sulfate is also produced