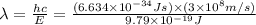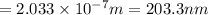Chemistry, 06.10.2019 05:30 kaylarae1930
Consider the following energy levels of a hypothetical atom: e4 −1.21 × 10−19 j e3 −5.71 × 10−19 j e2 −1.05 × 10−18 j e1 −1.55 × 10−18 j (a) what is the wavelength of the photon needed to excite an electron from e1 to e4? (b) what is the energy (in joules) a photon must have in order to excite an electron from e2 to e3? (c) when an electron drops from the e3 level to the e1 level, the atom is said to undergo emission. calculate the wavelength of the photon emitted in this process.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 09:30
Right anwser gets marked brainliest newton's discovery concerning how fast an object will change speed is the: 1st law 2nd law 3rd law universal gravitation
Answers: 1

Chemistry, 22.06.2019 15:30
What best discribes the relationship between wavelength and frequency in a electromagnetic wave
Answers: 1


Chemistry, 22.06.2019 21:30
What is happening when the water inside a kettle heats up and begins to boil
Answers: 1
You know the right answer?
Consider the following energy levels of a hypothetical atom: e4 −1.21 × 10−19 j e3 −5.71 × 10−19 j e...
Questions



Mathematics, 30.10.2019 22:31















Computers and Technology, 30.10.2019 22:31


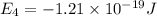
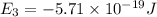
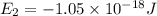
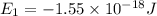
 to
to  .
.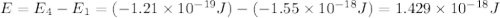
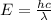
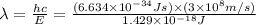

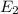 to
to 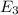 .
.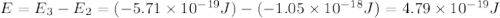
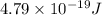 is the energy a photon to excite an electron from
is the energy a photon to excite an electron from