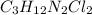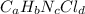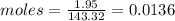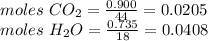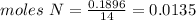Chemistry, 04.10.2019 20:20 estrellagutierrez12
Compound x contains only carbon, hydrogen, nitrogen, and chlorine. when 1.00 g of x is dissolved in water and allowed to react with excess silver nitrate, agno3, all the chlorine in x reacts and 1.95 g of solid agcl is formed. when 1.00 g of x undergoes complete combustion, 0.900 g of co2 and 0.735 g of h2o are formed. what is the empirical formula of x?

Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 06:30
Type the correct answer in the box. spell all words correctly.what is the correct term for living the most sustainable life you can within your current circumstances? when your are being as sustainable as you can within your current lifestyle, you are said to be sustainability.
Answers: 3

Chemistry, 22.06.2019 14:50
Which of the following is most likely true about water in chemical solutions?
Answers: 1

Chemistry, 22.06.2019 15:30
The identities of substances are the same before and after which type of change
Answers: 1
You know the right answer?
Compound x contains only carbon, hydrogen, nitrogen, and chlorine. when 1.00 g of x is dissolved in...
Questions

Social Studies, 20.09.2020 04:01






History, 20.09.2020 04:01



English, 20.09.2020 04:01





English, 20.09.2020 04:01

History, 20.09.2020 04:01

Mathematics, 20.09.2020 04:01


Mathematics, 20.09.2020 04:01