
Astrip of magnesium metal having a mass of 1.30 g dissolves in 150. ml of 5.00 m hcl (specific gravity = 1.10); products of the reaction are magnesium chloride and hydrogen gas. the hcl is initially at 22.0°c, and the resulting solution reaches a final temperature of 52.0°c. the heat capacity of the calorimeter in which the reaction occurs is 345 j/°c. calculate δh (in kj/mol) for the reaction under the conditions of the experiment, assuming the specific heat of the final solution is the same as that for water (4.184 j/g°c).

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 21:30
What is another way to determine mass times acceleration?
Answers: 1

Chemistry, 22.06.2019 23:00
What is the oxidation state of each individual carbon atom in c2o42−?
Answers: 1

Chemistry, 23.06.2019 05:00
Match each term to its description. match term definition excess reactant a) reactant that can produce a lesser amount of the product limiting reactant b) amount of product predicted to be produced by the given reactants theoretical yield c) reactant that can produce more of the product
Answers: 3

Chemistry, 23.06.2019 11:00
Find the enthalpy of neutralization of hcl and naoh. 87 cm3 of 1.6 mol dm-3 hydrochloric acid was neutralized by 87 cm3 of 1.6 mol dm-3 naoh. the temperature rose from 298 k to 317.4 k. the specific heat capacity is the same as water, 4.18 j/k g. a. -101.37 kj b. 7055 kj c. 10,1365 kj
Answers: 1
You know the right answer?
Astrip of magnesium metal having a mass of 1.30 g dissolves in 150. ml of 5.00 m hcl (specific gravi...
Questions


Biology, 24.06.2021 19:00

Mathematics, 24.06.2021 19:00


Mathematics, 24.06.2021 19:00


English, 24.06.2021 19:00



Mathematics, 24.06.2021 19:00

Social Studies, 24.06.2021 19:00





Social Studies, 24.06.2021 19:00



English, 24.06.2021 19:00

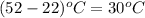
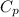 = 345
= 345 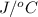
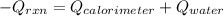
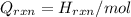
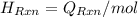
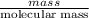
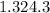
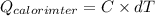
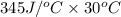
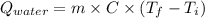
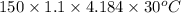
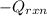 = (20710.8 + 10350) J
= (20710.8 + 10350) J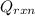 = -31060.8 J
= -31060.8 J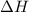 of the reaction as follows.
of the reaction as follows. 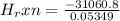
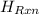 = -580684.24 J/mol
= -580684.24 J/mol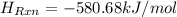 (as 1 kJ = 1000 J)
(as 1 kJ = 1000 J)

