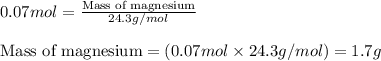Magnesium (used in the manufacture of light alloys) reacts with iron(iii) chloride to form magnesium chloride and iron. 3mg(s) + 2fecl₃(s) → 3mgcl₂(s) + 2fe(s)a mixture of 41.0 g of magnesium ( = 24.31 g/mol) and 175 g of iron(iii) chloride ( = 162.2 g/mol) is allowed to react. identify the limiting reactant and determine the mass of the excess reactant present in the vessel when the reaction is complete. a) limiting reactant is mg: 67g of fecl₃ remains. b) limiting reactant is mg: 134 g fecl₃ remains. c) limiting reactant is mg: 104 g fecl₃ remains. d) limiting reactant is fecl₃: 1.7 g of mg remans. e) limiting reactant is fecl₃: 87.2 g of mg remains.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 08:00
Define dew point. i am writing this part to be able to ask the question
Answers: 1

Chemistry, 22.06.2019 19:20
The equation picture below shows which type of nuclear reaction u 235 + n x e 134 + sr 100 + 2n
Answers: 1

Chemistry, 23.06.2019 01:00
The primary products of complete combustion of fossil fuels are a. carbon dioxide and water b. methane and water c. carbon monoxide and water d. carbon dioxide and carbon monoxide
Answers: 1

Chemistry, 23.06.2019 04:00
If you are told to get 100 ml of stock solution to use to prepare smaller size sample for an experiment, which piece of glassware would you use?
Answers: 1
You know the right answer?
Magnesium (used in the manufacture of light alloys) reacts with iron(iii) chloride to form magnesium...
Questions

Biology, 06.04.2021 04:00

Mathematics, 06.04.2021 04:00

Business, 06.04.2021 04:00

Mathematics, 06.04.2021 04:00

Mathematics, 06.04.2021 04:00

Mathematics, 06.04.2021 04:00

Social Studies, 06.04.2021 04:00



Mathematics, 06.04.2021 04:00




Physics, 06.04.2021 04:00



Biology, 06.04.2021 04:00

Mathematics, 06.04.2021 04:00

Mathematics, 06.04.2021 04:00

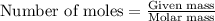 .....(1)
.....(1)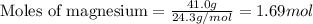
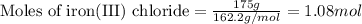
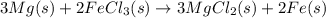
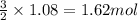 of magnesium
of magnesium