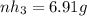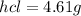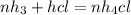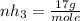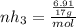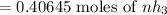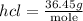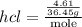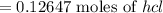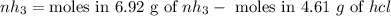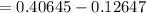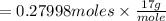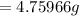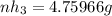Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 01:00
Which statement correctly describes potassium iodide, ki? there is a one-to-one ratio of potassium ions to iodide ions. potassium gains electrons and iodine loses electrons during the reaction. the lattice is held together by potassium anions and iodide cations.
Answers: 1

Chemistry, 22.06.2019 16:00
Click the button that shows the correct relationship of the electron affinities of the elements sodium and phosphorus. sodium’s electron affinity value is more negative than the electron affinity value of phosphorus. phosphorus’ electron affinity value is more negative than the electron affinity value of sodium. this information cannot be determined using the periodic table. answer is b on e2020.
Answers: 3

Chemistry, 22.06.2019 17:10
In which block of the periodic table is uranium (u) found? s blockd blockp blockf block
Answers: 1

Chemistry, 23.06.2019 00:30
How many moles of co2 are produced during the complete combustion of 3.6 moles of c2h6
Answers: 1
You know the right answer?
Ammonia rapidly reacts with hydrogen chloride, making ammonium chloride. calculate the number of gra...
Questions



Geography, 15.10.2019 05:40

History, 15.10.2019 05:40




English, 15.10.2019 05:40

Mathematics, 15.10.2019 05:40

Mathematics, 15.10.2019 05:40

Social Studies, 15.10.2019 05:40



Biology, 15.10.2019 05:40

Computers and Technology, 15.10.2019 05:40


Social Studies, 15.10.2019 05:40

Chemistry, 15.10.2019 05:40

Health, 15.10.2019 05:40